Over the past several years, I have increasingly been hearing about an oral anti-androgen called bicalutamide (brand name Casodex). While originally approved to treat prostate cancer in 1995, it is becoming a popular option in treating female pattern hair loss (FPHL).
Bicalutamide is a nonsteroidal antiandrogen (NSAA). It is also called a selective androgen receptor (AR) antagonist. Among the skin and hair conditions that it is being used to treat include acne, hirsutism and female pattern hair loss. Bicalutamide was originally derived from a structural modification of flutamide.
Update: August 2023 — A new paper from Brazil on treating female pattern hair loss covers bicalutamide in detail.
Update: April 2023 — From yesterday’s ISHRS Webinar:
Thanks to @ISHRS for the invitation to talk about #bicalutamide to treat female pattern #hair loss. A great meeting which covered some useful ground on upcoming #treatment options for male and female pattern #hairloss #alopecia areata as well as other conditions. pic.twitter.com/XFJfiPEPJO
— Dr Bevin Bhoyrul (@DrBevinBhoyrul) April 27, 2023
April 19, 2023
Bicalutamide for Female Pattern Hair Loss
A number of recent reviews, studies and presentations conclude favorably in regards to the use of oral bicalutamide to treat female pattern hair loss (FPHL).
- In 2022, researchers from Brazil reviewed various studies and concluded that oral bicalutamide seems to be a promising option to expand the arsenal of FPHL treatment. They do point out the need for clinical trials to establish the ideal dose and efficacy of the drug.
- At the 2022 ISHRS conference, Dr. Sergio Vañó Galván discussed two “new medications” to treat hair loss: bicalutamide and clascoterone (aka Breezula).
- Moreover, at the same conference, Dr. Alba Gómez-Zubiaur had a presentation titled: “Mesotherapy with Bicalutamide: A New Treatment for Androgenetic Alopecia.” In April 2023, he published a study on this interesting mesotherapy delivery method for bicalutamide. Make sure to also read my post on mesotherapy and dutasteride.
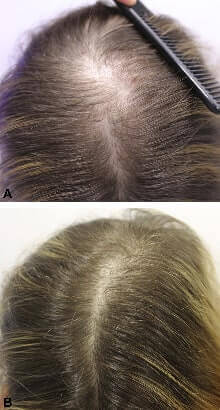
- In 2020, a group of Spanish researchers retrospectively reviewed all patients who were receiving oral bicalutamide (OB) for FPHL at their hair clinic between February 2018 and February 2020. A total of 44 women receiving OB 25-50 mg daily were included. They concluded that OB had favorable hair growth results and a very good safety profile, even when used in combination with oral minoxidil or spironolactone. One of their patient’s before and after photo is shown at the top of this post.
- In 2020, a team from Australia led by Dr. Rodney Sinclair reviewed the safety results in 316 women treated with bicalutamide. Doses ranged from 5 mg per day to 50 mg per day. The vast majority of them were also using oral minoxidil or spironolactone. In general, the safety profile was excellent and the drug benefitted female androgenetic alopecia (AGA) patients. The few patients who got a mild elevation in liver enzymes saw no adverse symptoms.
Side Effects
Bicalutamide has no diuretic effect and it does not cross the blood brain barrier. In comparison to another popular anti-androgen called flutamide, bicalutamide has a much lower rate of liver toxicity. Moreover, unlike the former, the latter does not affect serum luteinizing hormone and testosterone levels.
Bicalutamide can cause a mild elevation of liver transaminases (enzymes), peripheral edema and gastrointestinal complaints in a very small percentage of users. These side effects are usually reversible. Overall, this medication seems to cause fewer side effects than Spironolactone, the most popular anti-androgen that is used for reducing hair loss.
Note that bicalutamide is often used as an antiandrogen in feminizing hormone therapy for transgender women. And also as a puberty blocker in adolescent transgender girls.
In 2017, the combination of bicalutamide and a birth control pill was evaluated in a phase III clinical trial for the treatment of severe hirsutism in women with PCOS. The combination was found to be significantly more effective than a birth control pill alone.
https://interestingengineering.com/science/new-study-decodes-biological-cell-mechanisms-behind-gray-hair
Thanks Aleks. I got that in a few of my alerts and updated the grey hair post and republished.
Do you know any doctor in Sweden/ rest of Europe who prescribes Bicalutamide for FPHL?