Mallia Therapeutics (formerly MalliaBioTech) is a Germany-based company that is developing a novel hair loss treatment based on a soluble version of the CD83 protein (i.e., sCD83). Co-founder Dr. Alexander Steinkasserer authored a paper in September 2022 that found that hair follicle formation and wound healing are improved in CD83-treated mice.
The bottom of this post is my original 2021 write-up about this company, with new updates on top.
Update: September 15, 2025
Recently, someone from a company that handles Mallia Therapeutics’ communications e-mailed me the below update:
“I wanted to share a quick but substantial update on Mallia, which you last covered back in May. Since then, Mallia has undergone a strategic reorganization and is now operating as a holding company, Mallia Innovations GmbH, with two subsidiaries focusing on therapeutic and cosmetic applications of its sCD83-based technology platform.
The therapeutics arm, Mallia Therapeutics GmbH, continues to advance MAL-856, a drug candidate based on sCD83, aimed at treating various forms of alopecia as well as wound healing. The company is planning to advance the therapeutic candidate toward clinical trials.
At the same time, Mallia has launched a second subsidiary, Mallia Aesthetics GmbH, to develop innovative cosmetic applications for hair growth based on a related molecule, MAL-838, which is also derived from sCD83. Their focus is on establishing a line of hormone-free, science-backed cosmetic products under the name 8T3 that are based on the mode of action of sCD83. The launch of the first 8T3 products is planned for 2025, with the goal of making advanced, research-driven solutions accessible to both professionals and consumers.
To support the build-out of both subsidiaries, Mallia recently completed a 5.5-million-euro seed financing round. The capital will primarily be used to bring the cosmetic product line to market while continuing development work on the therapeutic candidate.
The company has also formalized two advisory bodies. A corporate Advisory Board now includes Jens Holstein (former CFO of BioNTech), Dr. Alexandra Ogilvie (dermatologist and digital opinion leader), and Dr. Ulrich Dauer (serial biotech CEO). In parallel, a Scientific Advisory Board has been established, featuring leading experts in dermatology, translational research, and hair biology, including Prof. Dr. Ulrike Blume-Peytavi, Dr. Claire A. Higgins, Dr. Gillian Westgate, Dr. Geert Cauwenbergh, and Prof. Dr. Franklin Kiesewetter. These groups will provide strategic and scientific guidance as Mallia advances both clinical and consumer-facing programs.
With the new structure, Mallia wants to deliver on its original therapeutic vision while expanding into the cosmetics space for rapid market entry.”
Update: May 28, 2025
Mallia Therapeutics: 8T3 for Hair Regeneration
It seems like Mallia will soon release two cosmetic hair loss products that are based on the ingredient MAL-838 (a formulation of the soluble CD83 protein):
- 8T3 Essentials for at-home use.
- 8T3 ProLine for profession use.
Note that they also have a page on MAL-856, which is described as “a structural variant of the extracellular domain of the membrane bound form of CD83 (mCD83).” Both alopecia areata and androgentic alopecia are mentioned on that page. It is unclear whether the company still plans to conduct clinical trials and release a stronger non-cosmetic product in the future.
- Mallia Therapeutics will participate at the 2025 annual meeting of the European Hair Research Society (EHRS) in Warsaw, Poland from May 29 to 31, 2025. Their presentation is titled “Soluble CD83 as a novel therapeutic approach for the treatment of alopecia.”
- Also of note, in February 2025, Mallia Therapeutics and Northway Biotech (Lithuania) announced a partnership for the development of the production process and manufacturing of Mallia’s soluble CD83 protein (sCD83). Northway is a biologics Contract Development and Manufacturing Organization (CDMO) with facilities in the US and Lithuania.
Update: June 18, 2024
Mallia Therapeutics expects to start 150-person clinical trials at the beginning of 2025.
Update: July 11, 2023
Mallia Therapeutics Secures Seed Funding
Mallia Therapeutics (previously MalliaBioTech) finally has a website. Moreover, the company just announced that it has secured seed funding. They now plan to raise Series A funding prior to beginning clinical trials.
The company is developing a soluble CD83 (sCD83) based topical treatment of hair loss.
This sCD83 has an immune-modulatory function and induces hair growth via a dual mode of action:
- It induces an anti-inflammatory environment around the hair follicles via regulatory T cells (Tregs). These interact with follicular stem cells and activate hair growth.
- It directly binds to follicular stem cells and induces the formation of new hair follicles. So sCD83 not only prevents hair loss and accelerates growth, but also induces the growth of new hair.
Moreover, this treatment will supposedly work for both androgenetic alopecia (AGA) and the less common alopecia areata (AA).
Key quote (slightly reworded):
“The company is led by world leading experts in the field of CD83, with more than 60 CD83-related publications and 20 years of relevant experience.”
November 4, 2021
A new biotech company in Germany named MalliaBioTech is working on a topical hair loss treatment based on the CD83 molecule.
Mallia Biotech, CD83 and Hair Loss
On October 27, the Friedrich-Alexander University (FAU) in Germany published an important page on their site titled:
“FAU project wins funding for remedies against hormone-related hair loss.”
An soluble CD83 based active ingredient newly developed at FAU leads to new hair growth. The FAU researchers from the Department of Immunomodulation and the Department of Dermatology at the University Hospital Erlangen received the m4 Award for this project on October 21, 2021.
So far, this CD83 (see gene card) based active ingredient has not shown any side effects in pre-clinical studies.
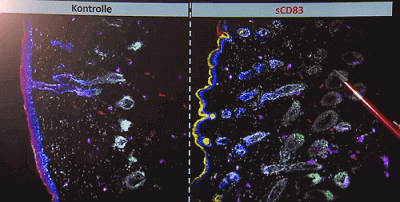
The research team is led by Dr. Alexander Steinkasserer, and the co-researchers include Dr. Dmytro Royzman and Prof. Dr. Carola Berking. Their new company is called MalliaBioTech, and it received EUR 500,000 via the m4 Award. It is officially called the m4 Award from the state of Bavaria.
“The new product has the potential to conquer the large unfulfilled market of hair loss.”
In contrast to existing hair loss treatments Finasteride and Minoxidil, this topical treatment based on a soluble form of the CD83 molecule:
“Stimulated the formation of new hair follicles and thus induces new hair growth.”
While the current pre-clinical work is in mice and yet to enter human clinical trials, this research represents yet one more new method to tackle androgenetic alopecia. We read about at least five such major new hair loss cure related discoveries every year, so reader skepticism is warranted.
When I first heard about CD83 for hair growth, I assumed it would be for alopecia areata. This is due to the frequent use of the word “immune” in tandem with CD83. However, the new German research clearly indicates that this treatment will also work for hormonal hair loss (aka androgenetic alopecia).
First order with promo code 600 euros.
There are results. I’m speaking in general, androgenetic alopecia is like a headache, some people will find aspirin helpful, some ibuprofen or something else, the same goes for medications or cosmetics for alopecia. I used KX 826 for quite a long time and gave up because I didn’t notice anything, some people it helped, some people it didn’t. Every human body reacts differently to a certain therapy, regardless of which medication was used.
Yeah, some people react differently, and it was going to be the same with this product, but the problem is the obvious manipulation with those before/after photos, it is insulting. If both had the same lighting and clarity, they would look similar.
I had hopes with this product. It is beyond demoralizing.
So it costs 750 € and, for now, the effectiveness is showed with 3 photos where two of them have used it for 12 months and all I see is hardly any change and a blatant manipulation playing with blur and lighting.
This is a joke. Another deception.
Where did you see 3 photos? I am seeing just 2 of the same person.
This is very few proofs of efficiency indeed. The price tag of 750 is therefore not justified. I am not in a hurry to order.
I have also noticed the blur and lighting effect. This is not serious.
Hairloss serum 8T3 is available for orders. Sticker price is 750 euros for 6-month supply. That’s $861 USD. The web page has 3 before / after photos, two of the gents are in their 60s. The photos are better than what I expected. Now the bad news – unless I am reading this wrong, they do not ship outside EU countries. Why dwarf your sales by not shipping to the US?
It probably has something to do with regulations. Try to use a shipping forwarder, maybe? Just a suggestion.
All that being said, unlike you, I am less than impressed with the pictures – keeping in mind the lighting conditions in the “after” photos. May I ask what exactly caught your attention?
I just think the price is too high.
I agree – but for some, this may be the only recourse against balding.
I presume this is more because they haven’t yet passed the regulatory requirements for the US market but I am sure they are currently working on that.
Webshop is here: https://8t3.com/en/8t3-essentials-hair-serum/MAL10000, with some rather modest before and after pictures.
I’d say there are some gains, but not really notable.
The product is available to buy now but price is really high. Also will only ship to EU countries.
Would be interesting to see if it works
They announced that sales would start in 5 days, but they did not announce the effectiveness of the cosmetics.
They can’t know the effectiveness.
They should know by now the effectivenes. They have two studies underway (for hair and for lash and brow). I hope they release some important data soon for basic trust.
4 more days till launch, boyos.
I reached out to Mallia’s customer care and got a response from Dr Steinkasserer:
– The 8T3 topical will be a liquid vehicle and not foam. It will also be propylene glycol free, great news for those of us with sensitive skin.
– I asked about human studies release date since all in vivo studies on their site have been on mice. Response: “We have performed extensive in vitro and in vivo studies with human hair follicles, which we are currently writing for publication. Once accepted, we will make it public.” So hopefully we will have those soon since the product is releasing shortly.
Their instagram account is now saying potenital release date of November.
I don’t know how this topical is supposed to work. They say it doesn’t penetrate the skin and therefore does not act systemically, and there’s an article on the European Biotechnology Magazine that says that sCD83 is a protein with a molecular weight of 45kDa (pretty far away from the 500 Dalton rule).
So, if it cannot penetrate the skin, how is it supposed to reach the hair follicle to activate its mode of action? I don’t understand. Also, if turns out the protein can reach the hair follicle, how is it supposed to not go systemic?
Found this on the Mallia Instagram. Confirms it’s daily application and confirms the price.
https://mallia-com.translate.goog/de/aesthetics/lp-haar-serum?utm_source=instagram&utm_medium=organic&utm_campaign=generisch&utm_content=haarserum&_x_tr_sl=auto&_x_tr_tl=en&_x_tr_hl=en-US&_x_tr_pto=wapp
I found the following unlisted competition page on the Mallia sitemap and got some details.
https://www-mallia-com.translate.goog/gewinnspielbedingungen-meta?_x_tr_sl=auto&_x_tr_tl=en&_x_tr_hl=en-US&_x_tr_pto=wapp
Some pictures of the starter kit are on their insta
8T3 by Mallia (@8t3_by_mallia)
From the pictures looks to be the starter kit is a 6 month supply and it’s applied once a fortnight maybe.
Also for a German specific email the 6 month supply will be €750
€750 is expensive if there is no clear efficacy.
Seems similarly priced and marketed as Cosmerna, and I imagine will be just as (in)effective.
Based on an overnight email, 8T3 hair serum is being released to those who subscribe to their email list as early as mid-October. I think I’ll give it a try. I’m twiddling my thumbs waiting for the next big thing, which I think is still at least 3 years out. So what the hell.
Looks like they are planning to launch in Oct. I just got an email about it with a 20% coupon code for ppl on the mailing list. So join if interested in trying it. i don’t see this update on the website yet though.
Hey Admin, it’s been a minute. Please tell me you have some JUICY news cooking for us. This year has been slow :/
I‘m not admin, but it’s worth following the HairDAO.
Unfortunately they are only active on Twitter and Discord (both difficult to participate).
Really pushing the research, new lab, new candidates etc. Next release is a liposomal topical Dut in October.
Don’t you mean decade?
Breezula phase 3 results were meant to be early H2.
News out today from the company.
https://www.eqs-news.com/news/corporate/mallia-aesthetics-mallia-to-present-scd83s-potential-for-supporting-hair-transplantation-at-the-fue-europe-meeting-in-madrid/2cc84893-aada-4529-9475-95ae81852025_en
Thanks Meko! I added a link instead of the text you pasted. Worth a read.
I also got that as a newsletter – it’s interesting but also confusing.
I think good hair clinics have very high survival rates of grafts – would Mallia even make a difference? Or is it for better wound healing – sCD83s initial research was for that purpose.
I am also losing overview on the various different lines with the numbers and letters, I don’t know what is what and what is the difference between the cosmeceutical and the other stuff?
Still waiting for efficacy numbers…I really root for them and hope they are not a fraud like so many others before.
wouldn’t it make more sense for them to apply their drug to the donor area after extraction if their trying for wound healing and new hair regeneration?
How effective will the cosmetic be?
Mallia Aesthetics are doing two studies on their cosmetic line.
12 week Eyelash and Brow serum study with results expected in Fall 2025.
6 months Hair growth serum trial with no expected date for results.
Both studies are currently underway.
Source:
https://mallia.com/posts/mallia-aesthetics-fuehrt-zwei-verbraucherstudien-zu-haar-und-wimpernwachstum-mit-8t3-seren-auf-basis-von-scd83-durch
I was in contact with their customer service – they are very helpful actually.
They said product release is in Q4. All studies so far have been „ex vivo“.
It‘s interesting that they can release a product without any human data.
Dr. Steinkasserer himself mentioned in an interview that people tested it (inofficially obviously) and had „clearly more hair“ (sic).
I will try it definitely. I do think though it will be a failure eventually – it just doesn‘t sound like something that will provide significant results.
I did hear those rumors that it had unofficial tests and grown hair, nice to have that confirmed to have come from them. They must have done some safety tests and noticed some hair growth.
Cheers for sharing.
Hey Falcon, I copied my comment from earlier this year including the link of the interview:
–
https://mallia-therapeutics.com/mallia-therapeutics-and-northway-biotech-announce-partnership-for-the-manufacturing-of-soluble-cd83-protein-for-hair-loss-treatment/
I‘ve just reread the article admin posted above – Dr. Steinkasserer said in that interview that 3 people tested the substance twice a week (topical application) for four months – he then showed pictures to the journalist stating „more hair, no question“. That sounds absolutely great, though how can they test it in humans in such a uncontrolled way and without any permission? I think that’s against every code of ethics in the scientific community…I am sure this happens all the time behind closed doors, but to mention that to a journalist in such a nonchalant way is rather unusual. They at least must be certain that it’s very safe.
I‘ve just reread the article admin posted above – Dr. Steinkasserer said in that interview that 3 people tested the substance twice a week (topical application) for four months – he then showed pictures to the journalist stating „more hair, no question“
Where is this article?
The link was in the article but admin must have taken it off.
Dr. Steinkasserer said 3 people tested the topical for 4 months, twice weekly.
Pictures were made which apparently showed „clearly more hair“.
Usually, I only remove important links if they no longer work (broken url) or if the post becomes too large from regular updates. Forgot if I ever removed anything here.
On a related note, the image in the post (from 2021) says that it is from a Mallia video. I can no longer find that video, and perhaps that link was removed if no longer working. Usually I would have a link to the video too.
Admin, do you have a day job? ;-) Appreciate the hard work you put in to keep this place ship shape as possible.
Sometimes I have full-time contract work, sometimes I do part-time work for old clients. Nothing in any job interests me as much as regenerative medicine (including hair regeneration).
I also appreciate everyone who tolerates this very old blog theme and the nuisance ads and affiliate links I have to spam :-(
This is the only active, interesting MPB hair loss related blog left, I visit three other “legacy” sites and they’ve essentially become dormant graveyards. I get most of my news on the topic from you Admin, keep up the good work!
I think they’ve been conducting research since 2001, so 24 years, the code of ethics means that someone publishes a medicine after a few months of research, not a cosmetic product after 24 years.
My hope is this with OS-01 will provide small gains to complement min and/or fin as we wait for better solutions.
Haha hair loss research feels doomed these days. Now we come from cherry picking trial data like Pelage and ET02 to “clearly more hair” in in official trials.
Minor update:
https://mallia.com/posts/mallia-aesthetics-achieves-key-regulatory-steps-for-novel-scd83-derived-ingredient-for-hair-growth
haha I think we posted the same thing!
I think your link is from Coegin/Follicopeptide?
New press release, the cosmetic product passed safety tests and is on track for Q4 release.
https://www.coeginpharma.com/investor-relations/press-releases/?slug=coegin-pharma-provides-status-update-ahead-of-the-upcoming-c-59278
Thanks Admin, this is good news IMO!
Esteemed scholars do not put their faces on things they are not confident may have some ground. She must have seen something before accepting the job… I mean data, (quantitative) assessments, possibly pictures… come on!
Dr. Claire Higgins is among 5 appointed as scientific advisors by Mallia:
https://mallia.com/posts/mallia-appoints-five-leading-international-experts-in-hair-science-to-its-scientific-advisory-board
Looks like Mallia are preparing their webshop but primarily for the DACH region, but it should be possible to import into other countries.
And their professional version looks to be only going to Clinics in the DACH region as well.
https://www-goingpublic-de.translate.goog/life-sciences/mallia-setzt-auf-prominente-koepfe-beirat-marktstart-haarwuchs/?_x_tr_sl=auto&_x_tr_tl=en&_x_tr_hl=en-US&_x_tr_pto=wapp
They are loud with all their announcements and releases.
But they lack the most important information for every potential customer: effectiveness.
Give me the numbers, give me the pictures.
I am not saying their product lacks effect, I hope it has some or even a lot. But until they release their results I cannot take them serious. To talk about the opening of a webshop and production and so forth but NEVER about the effects is a choice and doesn’t give me trust, at all.
This community has enough experience with snake oil cosmeceuticals and will crucify Mallia online if this doesn’t work. I hope their board realizes that.
This is Dr. Brotzu coming to you via Yoda, no need for numbers or pictures Benjamin, that’s so 1980’s. I will highly recommend this product and will use my esteemed medical credentials to vouch for it.
What you said is absolutely to the point.
“ 8T3 stands for modern hair regeneration that offers more than just cosmetics. With evidence-based formulations and the innovative ingredient MAL-838, 8T3 directly targets the root of the problem – and accompanies people on their way back to healthy hair and a strengthened self-image. Whether as the highly concentrated 8T3 ProLine for professional users or the user-friendly 8T3 Essentials for everyday use – 8T3 combines scientific precision with a noticeable effect.”
Hate how this sounds like just another new L’oreal junk product.
Of course, since they’re not able to come with any solid data about efficacy, they have to bamboozle with marketing guff. The sad thing is that many people will still buy this stuff in vain hope of results. I learnt my lesson with cosmerna (and now unfortunately by the looks of things Niostem).
You are most likely right. Still gonna try it.
You miss 100% shot you don’t take.
That’s all together quite confusing.
Why not show pictures of actual hair growth or at least numbers? They‘re releasing this year after all.
Would be perfect to hype up the product.
On Linkedin, the managing director of Mallia Dr, Anne Asmuss just responded to someone asking as to when the product will come to market with the following statement:
“Coming soon. Watch out for end of 2025.”
A reader sent me the screenshot, but no link. Their product range is also a bit confusing. The products page url that I had in this post also no longer works (archive below):
https://web.archive.org/web/20250604155218/https://mallia.com/aesthetics/products
Also:
https://mallia.com/posts/mallia-secures-european-patent-covering-scd83-for-hair-growth-and-wound-healing
https://mallia.com/posts/mallia-to-showcase-innovative-scd83-based-approach-for-hair-growth-at-hairs25-symposium
Looks like the December rumor is closer to reality than the October rumor.
Also it seems the links are now:
https://mallia.com/aesthetics/brands
https://mallia.com/products
The link to the LinkedIn post from Mallia
https://www.linkedin.com/posts/mallia_live-from-hairs25-in-augsburg-today-activity-7344086226632617984-jOvv?utm_source=share&utm_medium=member_desktop&rcm=ACoAAAZlk2kBLdhB72auJIhEzdWK9KIkkJjU0LI
Mallia Aesthetics are gonna be having a presentation at the hair and science symposium on the 26th of June.
https://www.webdisclosure.com/press-release/mallia-aesthetics-mallia-to-showcase-innovative-scd83-based-approach-for-hair-growth-at-hairs25-symposium-nHQ5DYGoKQa?utm_source
Chances are it’ll be the same data as the previous talk that was for Mallia Aesthetics. But maybe we’ll get a better idea of release date.
If anyone interested, I emailed Mallia when the new hair loss products will be available for purchase. The answer was most likely by December 2025. I truly hope it would be earlier. This and Fol005. Wishing for the best.
Thanks John. yeah it is still further away. But combining those two might give some decent results, who knows…
I guess we have to wait and try.
Hmmm, less than a month ago they were saying Oct according to a previous post.
Newsletter Update (if interested): Mallia Secures European Patent Covering sCD83 for Hair Growth and Wound Healing.
Newsletter Update:
Malia has received 5.5 millions of funding and is preparing market entry in 2025!
It’s becoming confusing. It’s now 3 companies and they managed to gather 5,5 million – but that money isn’t going into trials at all?
So what‘s the difference between the 2 compounds in terms of efficacy? As per their website one is “for hair loss“ (MAL856) and one is “for hair growth“ (MAL838)…what?
I hope they realize that if they sell a neutered version with no or small effects, they gonna get annihilated online by their customers and then it‘s game over.
CosmeRNA was gone as quick as it appeared.
If they were going the cosmeceutical route, they were never serious to begin with.
You know they can have the best treatment in the world in their hands but unless they pass the drug trials the law prevents them from making any therapeutic claims, the cosmoceutical route thus being just a loop in the system?
Clinical trials aren’t just some empty formality, they’re what confirm whether the drug is the best treatment in the world. Without them you have no evidence.
Who said clinical trials are empty formality?
Thank you, OTOH, for repeating what I was saying to begin with: you have no evidence – by law, you are not allowed to make any claim.
Tried to get more clarity on these aspects…no answer back. They are making this exceedingly confusing.
Yeah I hope they know what they‘re doing. I want to see pictures or at least numbers of their topical. Generalized claims (“clearly more hair” quote Dr. Steinkasserer) are nice but not relevant.
I still believe in their product – the Universität Erlangen has an excellent reputation. They don’t produce grifters.
I thought that about Max Planck, which is what spurred me to spend €1k on Niostem…..
Oh man I hear you. The founder of Niostem really was a prof at Max Planck.
Well at least Dr. Steinkasserer is still an active researcher and professor at Uni Erlangen. I doubt somebody like that wants to ruin his reputation.
We will see. There has to be a significant effect in sCD83, everything else would baffle me.
FWIW: I’m a 1-year user of Niostem, nothing else.
I did gain hair at the hairline and temples. The rest of the scalp, as a diffuser: either maintenance or slow-slow decline.
I had used 0.2- 0.25% topical Fin in Trichosol for over 2.5 years and it delivered less. Stopped Fin like 2 months before starting Niostem.
I think we will get something good out of this.
If the cosmoceutical didn’t do anything, they would not launch it. I incline, knowing the law, that they are not allowed to say more than “hair growth stimulator” – exactly what they could slap over a bottle of Rosemary oil.
Good results -> extra funding (buyers, would-be investors) -> launch the medication.
It’s possible (and I’m runnind wild here) they slightly tackled/modified MAL-856 to something else just so that they can sell it as a different product in 8T3. This would be the best scenario.
Slightly altering a molecule yet still maintain its active properties is done pretty much routinely.
If you take sCD83 and experience good results, how will you know it wasn’t a placebo effect?
Good point, and technically, in the strictest sense, correct. Not debating that in the slightest.
However, that assumes two things, IMO (and I don’t want to put words in your mouth):
1. The weaker one: that no sort of efficacy study whatsoever has been done on this compound (which we know is false).
2. That no matter how much of an improvement one sees, that can be attributed to a placebo effect.
If I were to show that one, two…5,000 follicles that were, upon inspection (under trichoscan), scarred…or simply did not exist there but popped into existence after 6 months of using the product (of course, I’m using an extreme example here, but bear with me) would I be reasonable to assert, past a certain threshold, that my results were just placebo?
Also, let me ask you something else: if they sell 8T3 “as is” (as far as proof goes), and then, while they make money on it as a cosmoceutical, the same mollecule (again, bear with me…i have no idea if they removed a Carbon atom between the two in actuality) gets the FDA stamp of approval…would the effect on one’s scalp be different once that leaflet switches claims?
This is my entire point when I said you could have (the unproven) cure to everything under the sun on your hands, yet you could not make claims (which is fair and necessary) until the FDA certifies that it indeed brings back the dead :) from their graves fresh and smelling like daisies.
I am not claiming this will be the case. I am claiming if it were, it would go exactly like I described as far as marketing claims.
All you can say is cosmoceutical = unproven. “Is for the birds”…well, that’s something unproven. Of course the burden of proof is on the other side, yet you’re also not making the distinction between “it doesn’t work” and “I have no idea if it will work or not”. The latter being the whole premise of starting trials to begin with :).
Hope this makes sense.
Not sure I fully understand, but I also didn’t express myself well that I was making a philosophical point, it’s epistemology, if a tree falls in the woods etc. if a drug isn’t proven to be effective then it technically can’t be. I say cosmeceuticals are a waste of time because the AGA market is worth billions, if a strong lead shows up, it’ll find funding one way or another as the profits are so big
What is the difference between MAL-838 and MAL-856? Is it a totally different product?
Per what I have in the post:
MAL-838 is a formulation of the soluble CD83 (sCD83) protein.
MAL-856 is a structural variant of the extracellular domain of the membrane bound form of CD83 (mCD83).
Here is a link with the details (in German)
https://www.eqs-news.com/de/news/corporate/mallia-sets-strategic-course-for-future-and-completes-e5-5-million-financing/7ce7584f-0e7c-4832-a9b9-56a0a55fa78d_de
Thanks John!
OK, I got the Mallia Newsletter today where they explain they split the company in Aestetics and Therapeutics part. So they bring Cosmetics soon this year already but still go the Drug way with trials.
That sounds promising.
With an increasing number of compounds that have passed safety tests becoming cosmetic products, can PP405 and ET02 also take this route and be launched in advance?
Cosmeceuticals for male pattern baldness treatment is for the birds. I don’t want serious companies like Pelage and Eirion to flake out and go that route, invest properly in a serious prospect, spend the money to take it through trials and prove its safety and efficacy and then market it and make billions,. Don’t play games and release some weak BS that didn’t have what it took to pass clinical development as a medicine.
Does anyone have any idea why they are pushing the product (Mal-856) down the cosmetic route and not down the clinical drug route? It seems like a double edged sword to me. Great that we won’t have to wait a decade or so for clinical trials, and we may actually get our hands on this within the year. But, if it was felt to be truly effective wouldn’t they wish to push the product down the clinical pharmaceutical route? Or is there still hope this could be efficacious?
Regardless I am very interested in the proposed mechanisms. Ever since I read about how calcineural inhibitors may cause hair regrowth in AGA, I’ve always felt this is a very under researched area for AGA.
They are going with MAL-838 on that route. MAL-856 is a different product.
Quite confusing.
Well thats nice to see its going fast but its a cosmeceutical so I am skeptical. I really wanna see results in pictures. I hope its better than Cosmerna or Koshine. Till today there is nobody in the world who has good results with these.
I asked the company Mallia about when the product will be available. This was the response:
We expect that the sales shop will be opened in about 2 months, and the official sales is expected to start in October.
Thanks Wouter!
Thanks, but I don’t get it: why open shop in two months and start delivery only in October?
Would be so nice to know whether we can, as John Does, get the “professional” (maybe higher-powered?) “professional” grade stuff.
I wonder what the tangible difference is between the ‘essentials’ and ‘pro’ lines… What’s most intriguing is that they state that the ‘essentials’ line is “tailored to individual needs”, which seems like a bold claim for a mass market product.
FYI…summary from link posted above by Admin: The 8T3 hair regeneration line is centered around the active compound MAL-838, which is a formulation of the soluble CD83 protein (sCD83). This protein has demonstrated promising results in preclinical studies for promoting hair growth. The sCD83 protein operates through a dual mechanism: Immune Modulation: sCD83 induces an anti-inflammatory environment around hair follicles by activating regulatory T cells (Tregs). These Tregs interact with follicular stem cells, promoting hair growth. Direct Activation of Follicular Stem Cells: sCD83 binds directly to follicular stem cells, stimulating the formation of new hair follicles. This not only prevents hair loss but also encourages the growth of new hair .
Excellent summary. Thanks Pinotq.
Completely new website:
https://mallia.com
Splitting up in “Therapeutics“ and “Aesthetics“.
“8T3“, a derivative of “sCD83“ is the name of the cosmetic that is gonna be released.
There’s no dates available, but it sounds like soon? Maybe even this year already.
This is something I am gonna definitely try, probably in combination with microneedling.
High hopes, low expectations. Admin? Your take?
I’m gonna try this as well.
Thanks Ben! Can you post the link where it says 8T3 is the name of the cosmetic?
I am skeptical that a cosmetic can do much, but will still be glad if they go the cosmetic route (since their previously announced trials seem to have been delayed)? They are presenting at EHRS on Friday. I updated the post.
Surely any drug that showed promise as a medicine would secure VC to take it through trials to approval? Therefore, anything that’s released as a cosmetic won’t be clinically effective by definition? Learnt my lesson with cosmerna…
Yeah absolutely. CosmeRNA is a joke. I expect the same weak results from Follicopeptide from Coegin Pharma. Though I disagree with your take on VC – I don’t think it‘s easy even for the truly promising approaches out there (see Stemson and Pelage).
I trust the highly esteemed researchers from Mallia, the used MOA, the university of Erlangen which has a extremely good reputation. Therefore I have higher hopes for Mallia.
Ready to be disappointed though ;-)
Thing is, how will you even know if it actually works in the absence of evidence based medicine?
100 % true. I don’t know if it works and how good it works.
But I have considerable trust in these guys.
I will try myself. As an Austrian it’s probably easier and quicker to obtain.
Fair enough, best of luck. But what I’m saying is even if you have results after using it, you can’t ever know if it came from the cosmetic or some other reason, but I suppose that won’t matter if you end up growing more hair while using it.
IT could happen that it would have an effect, albeit a small one. However combining it with other approaches might induce an even better effect.
I would like to try it and combine it with other treatments who knows how far I would come.
Do we know anything about ETA for arrival on the market?
I am thinking of sending an e-mail tomorrow.
Did they answer you?
Not to me. But they did to Wouter the comment is below this one.
https://mallia.com/aesthetics/products
here is lists two products coming soon
8T3 Essentials – available soon in our store.
Our line for at home
8T3 ProLine – available soon in our store.
Our line for professional users
Thanks!
I am also very intrigued! It helping with inflammation, regrowth, immuno-modulating, topical and isn’t hormonal is really all the things I’m looking for.
https://www.eqs-news.com/news/corporate/mallia-therapeutics-reports-on-novel-therapeutic-approach-for-treating-alopecia-at-the-annual-meeting-of-the-european-hair-research-society-ehrs/78d0a71d-1ad3-43e7-b58f-68ac85bb89b2_en
You never know if these things amount to anything, but always worth keeping an eye on.
Minor news:
https://mallia-therapeutics.com/mallia-therapeutics-and-northway-biotech-announce-partnership-for-the-manufacturing-of-soluble-cd83-protein-for-hair-loss-treatment/
I‘ve just reread the article admin posted above – Dr. Steinkasserer said in that interview that 3 people tested the substance twice a week (topical application) for four months – he then showed pictures to the journalist stating „more hair, no question“. That sounds absolutely great, though how can they test it in humans in such a uncontrolled way and without any permission? I think that’s against every code of ethics in the scientific community…I am sure this happens all the time behind closed doors, but to mention that to a journalist in such a nonchalant way is rather unusual. They at least must be certain that it’s very safe…
Anyhow, a great contender, and I still believe that sCD83 combined with microneedling can be an absolute gamechanger as I also went through their research regarding wound healing – which was the initial target for Mallia‘s molecule.
Top 5 hairloss company imho.
Thanks Ben.
Thanks Ben. Hopefully they are able to release it as a cosmeceutical in a few years.
I have no clue about timelines, they announced it could be marketed as a cosmeceutical, yes.
Sometime I think that is only to please potential investors, as it is a much quicker and cheaper route. If they have to finance 3 phases then I doubt they’ll survive.
CD83 is endogenous and probably not patentable – but its soluble version might be though. It’s supposed to be very safe…twice weekly topical application over 4 months had visible results. So daily over a longer period could be even better. Plus the from me suspected superior effect with microwounding/needling.
https://pmc.ncbi.nlm.nih.gov/articles/PMC9564224/
I‘ve been a little too pessimistic in my last post. A few updates and keypoints from Germany:
• new funding of 2 million € in two tranches
• will be used for production of sCD83
• sCD83 clearly induces new hair, not just reactivation of dormant hair
• sCD83 is well known with an excellent safety profile
• CD83 is a endogenous protein which guarantees a faster development and route to market. The release as a cosmetic is also possible. It‘s considered to be available within a reasonable time frame.
• Next step is human application in 2025
Hi Ben, where did you find this info? Do you have a link?
Yes sure!
https://www.goingpublic.de/wp-content/uploads/epaper/epaper-Life-Sciences-2-2024/#94
Page 94, it’s in German though.
Great, thanks Ben! … Thats OK I am German :-)
I think thats the most positive news I have read since a long time. Sounds all very encouraging. They clearly say it can come as Cosmetics and Trial starts in 2025. So my guess would be they plan to do a Phase 1 and try to marked a Cosmetics after the Phase 1. Other companies do it the same way like Olix for instance.
Yes, I’m cautiously optimistic too.
This could be a really effective treatment, especially if it is neogenesis (which would be completely novel; except Verteporfin or Scube3 possibly).
In this case you might accumulate gains with every treatment cycle. A combination with microneedling might enhance effects too, but that’s purely speculative from my side.
Enough of the dumb restrictions, where can I get my hands on this? And yes I’m willing to go to some third world country for it.
https://www.laborjournal.de/editorials/3043.php
Newish interview, nothing new though. They seem incredibly slow to me, as if they‘ve done nothing since 2021? If you can’t even set up phase 1 with 30 participants in 3 years, than all
hope is lost. Very unfortunate.
It’s a pity as Mallia’s tech sounds like a really good approach.
Me likey. Also of course hoping for more than 3x new hair follicle growth (3 x 0 is still 0…exaggerating a bit but you know what I mean).
Got this in my alerts:
https://mallia-therapeutics.com/
New website online. Haven’t had the time to read it all yet, but looks good so far. A clear, informative and official communication tool (in form of a website) builds a lot of trust imho.
Admin, maybe you can break it down for us.
Thanks Ben! They are yet to begin Phase 1 trials, but I still republished the post with the update. Interesting based on the team’s relevant and long-term CD83 experience.
Any1 seen this min/fin spray topical we have in the Uk? Un-Thin it’s called:
https://unthin.co.uk/
I understand how the process works, but really, there are thousands (perhaps hundreds of thousands) that would sign up for testing right now – and sign whatever was needed. Heck, they’d do a video staring whatever these folks wanted to ensure they couldn’t sue etc. Do the testing on humans already! I’ll never understand why it takes six to ten years to get to human trials. The red tape and approval process must be insane in some countries. It’s sure there is good reason for it but come on.
True! We would be faster if we buy it our selfs and try it like RU.
If this company said their treatment was contagious like covid they could fast track it and come out with a billion doses sent globally in less than a year lol. I don’t have hope for this company especially when they said 4 years till trials. Why even bother put a press release out of trials are half a decade away lol. Stupid. History repeats itself with mpb industry. The only hope I have is a company out of nowhere that we know nothing about comes out with a drug side effect of massive hair growth and they commercialize it. Just like min and fin. All the drugs that were created for mpb have failed miserably. Happy Friday all:)
Agreed. Have a great weekend mjones.
Well this situations seems similar. The researchers were testing a molecule to treat MS and stumbled across hair neogenesis albeit on mice.
“Clinical trails might start in 4 to 5 years”. Meanwhile, no hair transplant surgeons are willing to test FDA approved veterporfin on a patient.
Verteporfin has very limited potential use. Basically it’s only use would be to improve donor areas by stimulating follicle neogenesis post extraction. It doesn’t have a topical application. Right now there are a number of well known modalities for this such as ACell, PRP, PRF, etc. There are very few patients that would be willing to undertake the use of verteporfin for this, especially if they will also be paying for the surgery themselves. Most Hair transplant surgeons are interested in doing Hair Transplants for profit, not research.
BIG NEWS GUYS!
After more than a decade, Replicel has finally…
…named their dermal injector: DermaPrecise!
https://twitter.com/DermaPrecise
I’m optimistic that within the next 5-20 years we can expect an official name for RCH-01.
Holy they are on fire now!
Unrelated to hairloss at the moment but maybe of big interest in the future: Demis Hassabis founded a new company together with Google. Based on Deepmind‘s research regarding protein folding, Alphafold.
https://www.isomorphiclabs.com/
Fascinating!
Can they use the AI drug development system right away or does that have to go through FDA approval…?
Promising. I wish it wouldn’t take a decade or more like I’m sure it will to find out if it works on humans and, if it does, get it into our hands. These things take forever. Still, it’s good news. Something to add to the list of maybes.
Yeah even if it works won’t be available before what..15 years from now…i.can go hang myself.
If someone speaks German, please translate the scientific portions of the video at the bottom. Thanks.
I will try it.
Translation from the video:
Prof. Steinkasserer was actually researching for proteins to treat MS. They were surprised when they found out that the protein induced hair growth in mice. The animals got a higher hair density. Further research showed that they even created brand new hair follicles. 3 times more new hair follicles than the untreated mice. Right now they are testing the CD83 in humans. If they get the same positive effect of it, clinical trails might start in 4 to 5 years. Its gonna be topical.
That is everything important I guess. On the screen they show the hair follicles which are growing at the moment, induced by the protein.
I hope my English is good enough.
Thanks Georg! The 4-5 years start date estimate seems odd, but the 3x hair growth is good to hear.
Yeah but serioiusly everything does grow hair on mice. Its actually difficult to find something which doesn’t haha.
Its already strange that they still get money to research for growing hair on mice.
I hope so much that riken will be fast… But since they don’t even have found a partner for clinical trials…
This is great news because hair follicle neogenesis is a conserved process between mouse and humans. Traditional hair growth in mice is usually hair elongation and shaft thickness. Those are indicators researchers look for because Mice do not have androgenic alopecia . so it’s very very exciting that this molecule was able to induce hair follicle neogensis which is something we rarely if ever see .
Who knows… maybe just like finasteride… the best hair loss treatment twill once again come as an unexpected result while being tested for other morbidities.
Hi Georg – I don‘t think that Dr. Steinkasserer said they are testing in humans (that’s completely unrealistic right now and would be almost equal to a clinical trial) but something like „Humansystem“ (it‘s hard to understand). So in my opinion he means a traditional „in vitro“-testing, which is the logical next step.
I would add one thing: they said that completely new follicles emerged, and that in pretty big numbers – so I think we are talking about neogenesis.
The 4 – 5 years seem to be a very conservative timeline for a topical. I would expect something like 2 years maximum. See Samumed or Follicum. That’s why I find it very alluring, but completely uninteresting from a standpoint of availability.
Hey.
He is talking about testing it in human systems yes.
They figured out that the molecule triggers new hair follicles to grow IN MICE. They think first trials might be possible in 4-5 years.