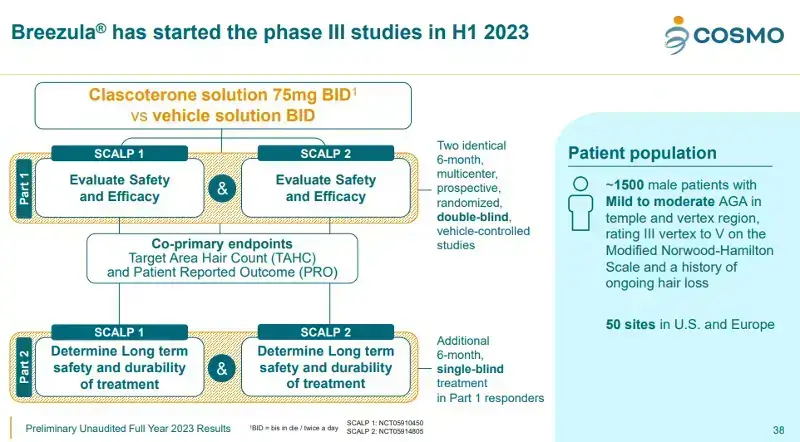
I have covered the novel androgen receptor antagonist Breezula® (clascoterone) for 11 years across numerous posts. The product was originally known as CB-03-01, and manufactured by a company named Cassiopea (Italy). The latter was acquired by Cosmo Pharmaceuticals (Ireland) in December 2021.
Cosmo Pharmaceuticals Announces Breakthrough Phase III Trial Results for Breezula (Clascoterone)
Earlier today, Cosmo Pharmaceuticals announced breakthrough Phase III trial 6-month topline results for Clascoterone 5% solution in treating male pattern hair loss (androgenetic alopecia). Subsequently, the company’s CMOPF stock price rose a significant 24%.
Interestingly, Cosmo did not use the word “Breezula” even once in the press release. It does not show up on the company’s pipeline page either. And the Breezula page on their site is not working at present. I really hope they do not change the name yet again. My blog post “categories” about this company and product are already too numerous.
Key notes from the press release:
- A massive 1,465 patients were randomized into the two identical clinical studies: Scalp 1 (NCT05910450) and Scalp 2 (NCT05914805). I covered these in detail here and here.
- Both studies reached statistically significant endpoints in Target-Area Hair Count (TAHC). The first reached 539% relative improvement compared to placebo; and the second reached 168% relative improvement to placebo.
- The safety profile across both studies for positive.
The way they present these numbers is a bit strange and may exaggerate the efficacy. And the large gap between 539% and 168% is very surprising. In the successful 6-month interim Phase 2 trial results that were presented in 2018, they chose to compare Breezula results with finasteride.
Cosmo will complete the required 12-month safety follow-up in spring 2026. Thereafter, Cosmo plans to promptly pursue parallel regulatory submissions in the United States and Europe.
“Clascoterone 5% topical solution is positioned to become the first topical androgen receptor inhibitor ever approved for AGA, subject to regulatory authorization.”
This development also bodes favorably for China-based Kintor Pharma’s androgen receptor antagonist KX-826 (Pyrilutamide). It is currently in Phase 3 trials. A number of other such products are currently in trials or have been released as weaker cosmeceuticals.
On a related note, Cosmo Pharma released Winlevi clascoterone 1% cream in 2020, and it has since become the number 1 most prescribed topical acne brand in the US. Some people were trying to use Winlevi off-label on their scalps in the past, but I suspect that the dose is too low. Moreover, scalp absorption of the cream might be totally different from scalp absorption of the solution.
Years ago, I mentioned how a hair transplant surgeon raved to me about Breezula based on a presentation (with photos) that he witnessed at an ISHRS conference. Fingers crossed that we finally have a third FDA-approved hair loss treatment by the end of 2027 (30 years after finasteride was approved, and 39 years after minoxidil was approved)!
I’ve seen CB powder on grey market sites. No idea how to make the product from that though. I’d be willing to try if there were clear easy instructions.
This seems underwhelming, even if both TAHC endpoints met statistical significance, the fact that one of the patient reported observations didn’t (“positive trends”) would suggest the actual visual improvement wasn’t particularly impressive.
Plus why would they be reporting in such a weird way if not to try and obfuscate the most important metric – significant cosmetic improvement.
More media coverage now:
https://www.bloomberg.com/news/articles/2025-12-03/cosmo-shares-soar-most-in-17-years-on-hair-loss-treatment-data?srnd=homepage-europe
https://www.investing.com/news/stock-market-news/cosmo-shares-surge-over-20-after-breezula-phase-iii-trials-hit-key-goals-4387715
Check out comments here:
https://x.com/HLcure2020/status/1996311255007768868
And on Reddit:
https://www.reddit.com/r/tressless/comments/1pcx8g0/breezula_6_month_topline_phase_iii_results/
This is great news. Cosmo has to realize that two additional treatments could be approved soon after this, nipping their heels. So I’m sure they are motivated to get this out as soon as they can.
Good news..but 12 months! Can’t they expedite it?
You mean it will be released in 12 months?
I’ve been waiting for over 30 years. 12 months is like 12 minutes to me. LOL.
I know, but I have been waiting over 20, and I don’t want to wait any longer.