
Veradermics (US) is working on a new extended-release oral Minoxidil tablet to treat androgenetic alopecia (AGA). The product is called VDPHL (as well as VDPHL01) and is currently in Phase 3 clinical trials. The top half of this post contains new updates in reverse chronological order. Also see my past posts on immediate-release low-dose oral Minoxidil and sublingual Minoxidil.
Update: October 16, 2025
Veradermics Raises $150 million in Series C Financing
Yesterday, we heard that Pelage Pharmaceuticals (US) raised a massive $120 million in Series B Financing. In 2026, they plan to begin Phase 3 trials for their hair growth product PP405.
Today, we have news that Veradermics has raised an even larger $150 million in an oversubscribed Series C Financing. And this is after they raised $75 million in 2024 from Series B Financing. Such numbers are unheard of in the hair loss world. Veradermics is already conducting multiple Phase 3 trials for their hair growth product VDPHL.
For context, in 2021, the esteemed RIKEN and Dr. Takashi Tsuji of Japan were finding it difficult to raise $5 million and even asked me to help them with the goal. And in 2025, Yunce Biotech (China) is finding it tough to raise just $2 million to move forward with their hair cloning work. See the most recent comments from reader “Jan Miedza” in that Yunce post. And in late 2024, the much hyped Stemson Therapeutics (US) folded because it could not raise sufficient funds. Many other hair loss companies that I have discussed on this site in the past folded due to lack of funds to proceed with tedious clinical trials and approval processes.
In the earlier mentioned press release from Veradermics, they do not mention any other ingredient besides extended-release oral Minoxidil in this VDPHL01 tablet. The do provide some great before and after hair growth photos from their Phase 2 trials:
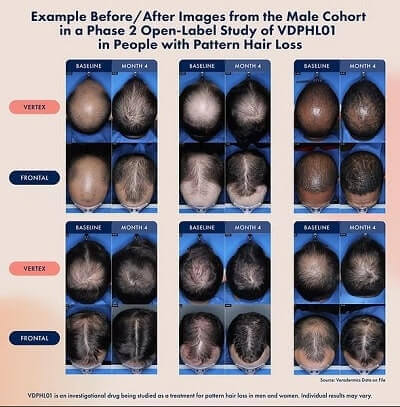
VDPHL Clinical Trial Updated Links
Enrollment links:
- 70 patients (male and female).
- Start date = 2024-07-08.
- Completion date =2026-08-28.
- 480 patients (males only).
- Start date = 2024-11-06.
- Completion date =2026-07.
- 552 patients (females only).
- Start date = 2025-07-25.
- Completion date =2027-03.
Update: September 29, 2025
Early Study Finds Extended-Release Minoxidil Grows More Hair
An optimistic summary of the results from the 20-person Phase 2 trial of VDPHL01, with the volunteers taking 8.5 mg VDPHL01 twice daily for 4 months. In the control groups, 33 patients received 5 mg immediate-release oral minoxidil once daily for 6 months; and 34 patients received 1 mL of 5% topical minoxidil solution twice daily for 6 months. Investigator Global Assessment (IGA) was the primary outcome that was measured, and the IGA ratings were made by blinded investigators.
The summary contains an interesting quote from Dr. Jerry Shapiro (who it is mentioned has a financial relationship with Veradermics):
“A longer time to sulfate is what we want, so if we keep the drug in the blood longer, there is greater sulfation and more activity.
Make sure to read my past post on Minoxidil sulfotransferase boosters and why Minoxidil requires sulfation in order to grow your hair.
Also of note, VDPHL:
“Offers an opportunity to maintain drug levels above those needed for therapeutic effect, but below those associated with cardiac adverse events.”
Update: September 21, 2025
Veradermics Extended Release Minoxidil for Hair Loss: Superior to Topical and Oral Minoxidil
Veradermics presented its smaller 20-person Phase 2 trial results at this month’s EADV Congress in Paris. A post about this on Instagram is causing some excitement. It has two slides in there, and I will paste the main points below:
- The doctor Congress attendee who made the Instagram post states that “Extended release oral minoxidil has superior increase in hair density compared to topical minoxidil and oral minoxidil. Phase 3 trials are underway.” So this confirms that VDPHL is extended release oral Minoxidil, even though Veradermics’ pipeline page still does not declare the key ingredient.
- 82% of VDPHL01 patients achieved moderate to great improvement versus just 20% of topical Minoxidil or low-dose oral Minoxidil users.
- There is superior efficacy (3.5 times higher IGA scores) in a shorter time frame (4 months versus 6 months) compared to “competitors”).

Update: January 2, 2025
Veradermics Phase 2/3 Clinical Trial Enrollment Link
Veradermics Phase 2/3 US clinical trial enrollment link is live. Their 40 plus locations are pretty widely spread across the country. Please note that we are not certain of the active ingredient(s) in this tablet. From the company’s patent, I previously guessed that it is an extended release oral Minoxidil, plus hopefully some other surprises (see bottom of this post). But it could end up being something totally different too.
The study involves 13 visits to a clinic over the course of 12 months. Participants will either get the new treatment or be part of the placebo group (that will still also get a tablet).
Update: December 11, 2024
Veradermics Raises $75 Million for Phase 2/3 Trials
Veradermics just raised $75 million in Series B financing (h/t “meko”). They have also initiated a Phase 2/3 trial for their lead candidate VDPHL01 for the treatment of androgenetic alopecia (AGA). The trial will enroll 480 patients across 44 sites in the US. Note that Veradermics also has an ongoing 20-patient Phase 2 trial for VDPHL. The company plans to report topline data from that Phase 2 study in the first half of 2025.
August 9, 2024
Veradermics VDPHL Tablet: Phase 2 Trials Begin
Veradermics is a US-based startup that is working on a new tablet to treat androgenetic alopecia (AGA). They just started Phase 2 clinical trials for VDPHL01 in male subjects with AGA. Only 20 patients are enrolled, and the completion date is listed as August 1, 2025. The trial will take place at Therapeutic Research’s center in San Diego, California. Note that VDPHL likely stands for Veradermics Pattern Hair Loss.
The tablet does not impact hormone levels as do dihydrotestosterone (DHT) inhibitors finasteride and dutasteride. Thus avoiding potential side effects. Veradermics’ CEO is a young dermatologist named Reid Waldman.
Modified Release or Extended Release Oral Minoxidil
The mechanism of action (MOA) and key ingredient(s) in this tablet are both confidential. However, when I searched through the company’s patent, it seems like the drug candidate will be a “modified release” oral minoxidil tablet. In the patent, they use the term “extended release (ER)”. Note that they do not use the term “sublingual minoxidil” anywhere in the patent.
Other Potential Ingredients in VDPHL
In the patent, they also have a massive list of 191 claims. Within that section, all of the following drugs are listed 11 times each:
- Setipiprant (11 times).
- Valproic acid (11 times).
- Cetirizine (11 times).
- Medrogestone (11 times).
For long time readers of this blog, setipiprant (and Kythera) will ring a bell. It caused so much excitement a decade ago. I cannot believe that the very optimistic 9-yr old audio interview with Kythera’s CEO is still online. Setipiprant is an oral antagonist to the prostaglandin D2 (PGD2) receptor.
I covered valproic acid and hair growth in detail in the past. Follica also has a patent that covers valproic acid and hair regrowth. Valproic acid activates the Wnt/β-catenin signaling pathway.
Cetirizine is a PGD2 inhibitor that has been shown to benefit hair growth even when used topically.
I have never covered medrogestone on this site before. Per Wikipedia, it is a progestin that is an agonist of the progesterone receptor and a weak anti-androgen. Progesterone is a female sex hormone that has beneficial properties towards hair growth.
Two questions:
1) Is it fair to say that if topical minoxidil has no effect at all (in my case) that this product won’t work either? I’d be happy with even a little more density.
2) Topical minoxidil isn’t that expensive, what price range do you expect it to fall into? (per month)
Nobody wants to fund a cure, its all drugs to keep you coming back for more. I have been saying that for years.
Hold on woofy97, what about the developments at kintor? What about pp405? Do you feel these scientists are not honestly working to find a cure? Maybe a “a cure” is also not a realistic goal, but do you feel these companies are not trying to better the options in this field?
At this point, it doesn’t matter if they don’t find a definitive cure, I just want them to find something that actually works, something that can regrow most of the hair I’ve lost. My hair is so thin that I now always have a hat on to cover the mess, and always wearing a hat really sucks.
Thats just fantastic news in the last days … btw they also update the pipeline
https://www.veradermics.com/pipeline
Now that I’ve heard about this 150 million for a drug that contains minoxidil, I’m much less optimistic about Pelage and its pp405… bad news.
My god.. what a negative mindset. Why can you not be optimistic about both? It’s objectively good news for both companies.
After 35 years of waiting it is hard to be optimistic. My best years are long gone.
I understand that Lorence, 35 years is a long time. I sometimes also look at an 6 year old kid with his balding dad thinking: you might be lucky when you grow up..
This kind of angers (actually a lot) that companies like Pelage and Veradermics have received +$100M investments but Stemson couldn’t…
Some people are never satisfied.
You were happy with the whole Stemson situation? Interesting.
I never followed it that closely, same with Yunce, seemed like pie in the sky to me. Certainly not dwelling on it days after announcements of $400M being invested in upcoming treatments.
If it were that great they’d get the money, not a big mystery there. Geez, you fan boys never cease to amaze me.
Boooooo!!!!
My thoughts exactly.
Fan boys or Boo LJ? :-) I could never figure out this whole “Anchorman” man approach to treatments. Anchorman as in the movie when the three news teams had a rumble. Who cares what horse wins the race, as long as one finally wins soon. As for treatments that die or can’t raise funding, there’s a reason, ultimately they don’t work for whatever reason, e.g. effectiveness, safety, cost, just like any other product.
Don’t know if I’d use the word fanboys, but I definitely do feel beggars can’t be choosers.
One day after Pelage, a new funding record is set?
https://www.fiercebiotech.com/pharma/veradermic-looking-tousle-hair-loss-market-locks-down-150m-series-c
That‘s insane. What’s going on?
Stemson would have needed what? 70 million?
And all that for oral minox…I only saw one patient photo and that was really underwhelming.
They must see better results too, otherwise you don’t fund this amount.
The results here look decent
https://www.veradermics.com/veradermics-announces-150million-seriesc-financing
I mean those pictures look fantastic. I’d be stoked to get those kind of results. Those are transplant level results. I have been on fin/dut and topical min (switched to sublingual Minoxidil since last week) for almost a year now and I am barely improved since baseline. I am still bald AF. If I could get results like this, it would be a miracle for me.
Decent?! Great, you mean.
Well, they don’t all look great do they?
They do to me.
Ok thanks, I didn’t see these ones before. I hope they are not curated.
If these are the standard results, then inject it into my veins :-)
Suddenly it doesn’t look so bleak anymore.
Hello,
When will new technology/medicine that is actually beneficial become commercially available? Even if it’s not like hair cloning, but something like Pelage that wakens the dormant follicles? So like not as exceptional as regrowing a full head of hair, but at least revives some of the follicles that remain dormant under fin+min. Thank you.
https://www.instagram.com/p/DO0bMQBDWsb/
Don’t these results look pretty good?
If what I’m reading is correct I’d be a buyer. Seems to help with dreaded MPB itch, scaling and pain as well. Thanks for posting LJ.
Yoda, to clarify I believe the improvements for scale and itch were monitored in the 2nd study of a jak inhibitor for patients with refractory LPP.
That being said the results for Veradermics based on the IGA improvement seems significant.
Does anyone know what IGA points mean in NW scale terms (if anything)?
Thanks for clarifying TP, my ADD with reading this stuff gets the best of me! Why I use phrases like “if I’m reading this correctly” not studying closely or hanging on every word, glad I at least got the improvement right….and still probably a buyer!
Thanking you here LJ! Have updated the post.
Any idea of a potential commercial release for this? Wild guess?
The trial ends in July 2026. Hope they release it in 2027.
Admin – I think someone posted in this thread earlier this summer that they tacked on additional Phase III time on ClinicalTrials.gov to project the final leg of the trial might end December 2026. But thanks for posting this. Great news on a bad day.
Crap, I was hoping within a year. Thanks though Admin.
Admin, what do you think will be better: sublingual (Sinclair) or Veradermics extended release?
Efficacy and side effects?
Technically you don’t have to wait for sublingual Minoxidil. I just got a sublingual min prescription from my derm to a compounding pharmacy. If you have a dermatologist that is open to it, compounding pharmacies can make sublingual Minoxidil troches/lozenges for you to put under your tongue now. No need to wait for Dr Sinclair, you can get this now.
Yes I know – it would be interesting though to know the differences between those two methods.
I dread side effects.
Ben, check out my new update on top of the post after refreshing the page. An interesting point about greater sulfation with extended release oral Minoxidil.
Hard to tell which one will be more effective at this stage betweel sublingual and extended release. But I would not be surprised if some people end up combining the two (like the 1 day dutasteride, 6 day finasteride type of strategy).
The only side effect from oral Minoxodil that I do not like is that it seems to have made some of my facial hair grow more rapidly, and maybe give me more fuzz on the forehead.
Darkprince, what country are you in and if in US what pharmacy? What mg did your Doc prescribe? Have you been on traditional oral min? Please keep us updated on your results with the compounded sublingual min!
I am in the US. My derm is using Noblesville compounding pharmacy in Noblesville, Indiana. Yes I was on traditional oral 5mg I believe a year ago. Had to stop after a few weeks due to chest pain. My dosage this time is 2.5mg sublingual. I will keep you updated for sure!
Much appreciated Dark, I hope it works well for you from a side effect standpoint and effectiveness. I’m on 10mg oral and compounded 8% topical 2x a day (other stuff in that too), not to mention Kirkland 5% foam in the very early in the am when drinking coffee, working out, etc before showering for the day. No sides but have lost some ground and looking for the next thing.
I’m surprised u lost ground on 10mg oral min Yoda.
Results:
https://www.reddit.com/r/tressless/s/1eMJl8JGVz
Looks pretty good!
Here is the link for female enrollment for Phase 3 (based on the timeline of the website not the study enrollment page). I wonder how it is hormonal if medrogestone is listed in the patent.
https://www.phlstudy.com/female/
This also works for the male recruiting: https://www.phlstudy.com/male/
Doubt it can be the VDPHL trial unless this guy did not tell them that he was also taking Finasteride:
“Oral Fin everyday and some type of minox (it’s a research study with a greater minox dosage than available in market or placebo…though I don’t think it’s the latter).”
https://www.reddit.com/r/tressless/comments/1ldcp6j/progress_pictures_18_ish_months/
The link in the Reddit-post doesn’t work anymore…maybe that guy thinks that the Veradermics tablet includes Fin?
I‘d like to see the effects of their treatment.
He (“Substantial_Lab_7131”) removed the original comment and great before and after results photos… but kept his comments underneath the post.
One reader asked him 13 hours ago if this was VDPHL01, and he answered “yep” an hour ago.
admin, did you see this, i think this could be a game changer.
https://www.reddit.com/r/tressless/s/oaojZP7Ykd
Thanks, had not seen it. The OP is in the trial and took those photos himself. Hopefully it is legit, though I am a bit suspicious since it is his first ever post on Reddit.
The results look better than just plain old oral Minoxidil.
By my estimates, Veradermics is about on schedule to be released right around the time Breezula is. Lots of assumptions there – like both get final approval. This is why I think Breezula is overly optimstic in their market share projections. And right on the heels of both of them could be Pelage if that gets approved (a year or two later). So, for some market entrants, it might be too late. But the more, the merrier. JMO
I like your optimism, it’s quaint. We should be so lucky if any/all get approved on schedule, would be ecstatic if even one does.
Mangoceuticals …. pharmaceutical-grade chewable
Is this the same approach as Veradermics or Samson Clinical?
https://www.accessnewswire.com/newsroom/en/healthcare-and-pharmaceutical/mangoceuticals-is-reengineering-the-self-care-blueprint-with-better-p-1018191
Another Phase 3 till 11/2026
https://clinicaltrials.gov/study/NCT06972264?cond=Androgenetic%20Alopecia%20%5C(AGA%5C)&rank=1
Pictures from the trial:
https://www.reddit.com/r/tressless/s/WeWnYKgwVt
That’s actually really disappointing. And this company got 75 mil funding? What the heck.
I mourn Stemson, what could they have done with the money.
Holy cow – I am very underwhelmed by those results. I hope that is not the typical ‘great’ responder. But then again, I only would use it as a bridge to Pelage (assuming either makes it to approval).
If it’s on Reddit it must be true.
So we could see this hitting the market by 2026/27? I for one, am very excited about this. oral Minoxidil on higher dose without side effects? Sign me up!
https://nypost.com/2025/02/07/health/new-hair-loss-treatment-being-tested-with-paid-volunteers/
Interesting, what’s the best estimate this would be commercially available if the clinical trials are successful?
I am moderately optimistic for this one.
I don’t know why they make such a secret of the ingredients – it is very likely Minox. Kinda ridiculous.
If they tweak the formula to more efficacy and less side-effects it’s definitely a win.
If it has the same results as regular oral Minox then I don’t know how they market this. That’s been available for many years for affordable prices.
It’s a bit mysterious.
I wonder if it will have more staying power than oral min…for me anyways. I had great gains with oral min about 5 years ago however they only held for about 3 years then lost ground. Not back to baseline, however not as thick and lustrous as it was at the pinnacle of my success. Pretty similar to my experience with topical min way back when and whenever I’d increase the concentration, an initial boost then backtrack. Hopefully they solve that part of the equation.
That would be a HUGE bummer. To psychologically think that you beat hair loss…and then have it come back again. Lame.
I’m in San Diego too Bro, Clairemont area. Keep us posted on your results. Oh, and just because that’s my outcome doesn’t mean other’s will suffer the same fate. Although our friend from down under Mr. SummyKim lost ground as well, I think under similar circumstances but could be wrong.
I believe that I’m part of this clinical trial, although I’m not sure if I’m phase 2 or phase 2/3. I am taking 8.5mg of VDPHL01 twice daily. I’ve been told that it’s a specially formulated version of minoxidil that is timed and/or extended release. I’m about to hit month 6, and my results have been slow but very promising.
From Veradermics’ LinkedIn:
Veradermics is at an exciting inflection point: after recently closing our Series B funding we are growing our Clinical Operations team to support our current Ph.2 and Ph.3 clinical trials. To help drive this next chapter, we’re searching for *2 Senior Clinical Trial Project Managers* to oversee trial execution and CRO partnerships.
Can we assume this Phase II / III trial will be the final trial? Seems odd if they could combine Phase II / III stages and make it the final trial unless there is no novel compound in it that hasn’t already been FDA approved.
A link to this story arrived in my email box this AM:
https://www.businesswire.com/news/home/20241211308760/en/Veradermics-Raises-75-Million-in-Series-B-Financing-and-Initiates-Phase-23-Trial-for-Hair-Loss
So considering early clinical and preclinical costs, they’ve invested $100 million to get approval for oral minoxidil(!). Crazy times, still, I suppose if it works….
See the bottom third of post.
Admin – what do you make of the money involved here? It seemed like it was a big deal when Pelage raised $16.75 million followed by $14 million, but Vermadermics has raised $75 million (or $100 million?). Should this be topping our lists of treatments to follow?
If it is just oral Minoxidil with a better delivery process, then I am not overly excited.
Exicure raised an immediate $25 million from Allergan in 2019, along with potential milestone payments of up to $265 million. It did not result in anything so far.
Admin, Exicure stopped their hair loss R&D over two years ago:
https://investors.exicuretx.com/news/news-details/2022/Exicure-Inc.-Announces-Termination-of-AbbVie-and-Ipsen-Collaboration-Agreements/default.aspx
This investment must have been the single biggest blunder in the history of hair loss research.
After that, they struggled to keep going and are barely alive with their other programs.
Thanks Ben.
On a related note the case of Kythera is really crazy. In February 2015, the company purchased global rights to Setipiprant (see my CEO audio interview link at the bottom of this very Verademics post).
Then Kythera itself got purchased by Allergan in June 2015.
Then AbbVie purchased Allergan in 2020.
Is this the same drug as someone earlier mentioned? the high dosage oral Minoxidil without side effects? i remember someone taking 2x 8,75mg without any sides. don’t remember if it was here or on Reddit. that would be amazing. 15/20 mg oral Minoxidil without side effects would be basically a (very hairy) cure.
I made a post on Reddit a couple of months back, but I deleted it. I’m currently taking this medication as part of the trials. 8.5mg 2x/day. It’s been 5 months and I’m not a hyper responder by any means, but I’m personally pleased with the results.
Great to hear. Are you experiencing any sides with it? Are the results better than finasteride?
Sides: I had headaches the first couple of weeks, but that went away.
Fin comparison: I had a prescription for oral fin a few years ago, but I didn’t take it consistently and stopped after a couple of months of seeing no results. So, I can’t make a fair comparison.
Interesting the screeners eliminated anyone with prior minox use so as not to skew the results how did u get in the study.
From the exclusion criteria it’s looks as though that was use only within the previous 4 weeks, but the more important question is how does anon know he isn’t taking placebo?
Thanks for publishing my comment and correcting my mistake admin
Phase 2/3 started
https://clinicaltrials.gov/study/NCT06724614?cond=Hair%20Loss%2FBaldness&rank=1
Thanks, I updated the post with trial links on top. Seems odd to start Phase 2/3 trials before Phase 2 trials have ended, but I think I have seen this in the past too.
Hopefully the tablet has all the 5 ingredients that I discussed in this post.
Mixed phases have always gotten me confused:
will this phase 2/3 be enough, or they will have to do another standalone phase 3 after that?
Extended release minoxidil could be reasonably simulated by dividing the daily dose and taking every 3 hours as that is the half life. A lot of work but it would be interesting to see if it’s effective.
So to sum things up, in the hair loss world, we still are battling this thing with the same old rebranded, repackaged dinosaur era minoxidil or finasteride. This sucks.
Interesting. Curious how it’ll be minox (to some extent) but without the sides. Not sure how they’ll pull that off and if people with heart issues will be able to take it (many with heart issues can’t take oral minox). Also, why only 20 people in the trial? For something like this, that doesn’t make sense to me but I’m no expert on the rules and guidelines of clinical trials.
So it’s oral Minoxidil? That’s unfortunate, I do not tolerate oral Minoxidil at all, even on 1mg doses.
As far as I remember some research for oral valproic acid causes hair loss and topically increases hair growth.