Update: October 20 2025
Absci to Begin Phase 1 Clinical Trials for ABS-201 in December 2025
In a new article about Absci’s ABS-201 hair growth injections that target the prolactin receptor, it is mentioned that Phase 1 clinical trials will begin in Australia in December 2025. The renowned Dr. Rodney Sinclair will oversee the trials at his Sinclair Dermatology clinic. Interestingly, Hope Medicine’s Phase 1 clinical trials for its prolactin receptor blocking HMI-115 injections also occurred in Australia at Sinclair Dermatology in 2022.
Of note, the Absci research team believes that ABS-201 could also cure premature greying by re-pigmenting the hair. This is based on their pre-clincial work on macaques, in which the animals’ hair reverted back from grey to black.
Key quote regarding Absci’s use of AI to develop this product:
“Using AI to develop the needle itself (in this case, the perfect antibody sequence) researchers were able to discover key antibody binding regions that could precisely target the prolactin receptor, thus ceasing hair loss. Using computer simulations, Absci was able to optimize certain drug qualities, including increased potency and decreased likelihood of negative immune response.”
Update: July 2025
The CEO of Absci posted the below Tweet recently. The before and after macaque monkey image in there seems identical to the image from Hope Medicine’s HMI-115 and its effect on macaques. Strange. The same image can also be seen in their 2024 R&D document. Both HMI-115 and Absci’s ABS-201 target the prolactin receptor to regrow hair. Hope Medicine has already completed Phase 2 clinical trials, while Absci is yet to commence any human trials.
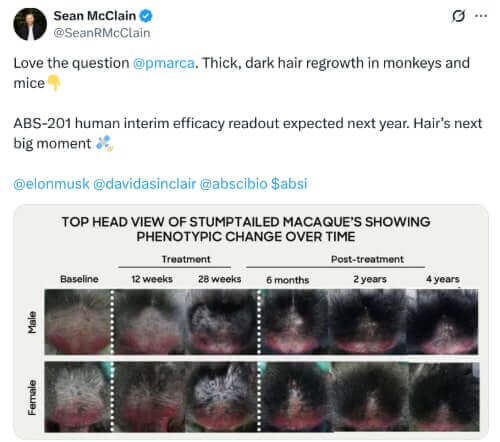
April 6, 2025
Absci (US) is a data-first artificial intelligence (AI) drug and biologic creation company that is unlocking novel treatments through the use generative AI. Most interestingly, one of the key products that they are focusing on is a hair growth treatment called ABS-201 that targets the prolactin receptor (PRLR).
I first heard about the company in January 2025 when Absci received a $20 million investment from chip manufacturer Advanced Micro Devices (AMD). Unlike virtually all new entrants in the hair loss world, Absci has a great website. However, it should be noted that the company originally started operations way back in 2011, under the auspices of founder and CEO Sean McClain.
In March 2025, Absci presented on the subject of AI in dermatology at the Dermatology Innovation Forum. On a related note, in 2022, I wrote a post on AI and machine learning for hair loss drug discovery.
Absci Presentation on ABS-201
Considering that Absci’s pipeline page shows that ABS-201 is yet to even enter Phase 1 clinical trials, I was reluctant to write this post. However a new video presentation (embedded below) that the company uploaded in February 2025 changed my mind. It is titled “Absci R&D Day 2024”. The portion devoted to ABS-201 and hair loss starts at 1:19:40 and lasts for over an hour.
Also check out the company’s case study page on ANS-201. Interestingly, in the notes to the key mechanism of action diagram, they mention that ABS-201 also has the potential to restore hair pigmentation.
Absci currently has over 77,000 square feet of space between:
- A state-of-the-art wet lab in Vancouver WA (US).
- An advanced AI research lab in New York City (US).
- A drug innovation center in Zug (Switzerland).
The company plans to begin Phase 1 clinical trials for ABS-201 in the first half of 2026. Their preclinical model demonstrated improved hair regrowth in comparison to minoxidil.
Absci ABS-201 versus Hope Medicine HMI-115

Note that Absci’s AI-developed ABS-201 is similar to Hope Medicine’s HMI-115 that is currently in Phase 2 trials. The latter prolactin receptor antibody treatment for male and female pattern hair loss was originally developed by Bayer (Germany) who received a patent for it in January 2019. A few months later, Hope Medicine announced a global licensing agreement with Bayer to advance the development of this monoclonal antibody to target the prolactin receptor.
In the above screenshot from Absci’s earlier mentioned recent presentation video, they elaborate on some of the key differences between ABS-201 and HMI-115.
Hopefully they can re-pigment the beard too and not just hair.
How often do the injections need to be administered? Or is it a one-time treatment?
Once quarterly.
That’s going to be a pricey affair. A few thousand euros/dollars a year?
Easily, especially if it also repigments grey hair.
The before/after pictures of the treated macaques would imply you will only need a few treatment sessions and then have results that last a few years.
Guess we’ll have to wait for the human trial results.
Phase 1 already starts in December 2025. That’s quicker than expected! They must be really convinced.
https://investors.absci.com/news-releases/news-release-details/absci-host-kol-seminar-abs-201-androgenetic-alopecia-program/
Yes, hopefully they can blaze through the trials like Veradermics and Pelage! I think it is quite promising.
I think neither Veradermics nor Pelage are particularly „blazing“.
Veradermics is using an already approved medicine and hence could skip one phase.
Pelage was founded in 2017. Research at UCLA happened even before that.
Both are going through clinical trials quickly. Veradermics initiated both p2 and p2/3 around the same time. Pelage finished p1, and p2a + extension in 2-2.5 years. Quite fast.
Another candidate to watch closely. I would be already extremely happy if we finally have a drug to reverse my premature greying that started when I was 18. Fingers crossed.
image is exactly same of Hope medicines .. it’s doubtful. how can they use the same image for their promotion or exhibit.
Sorry, that’s clearly the photo of HopeMed (or Bayer if you want).
HopeMed is probably finished with phase 3 before Absci starts their phase 1. They are a good 5 years ahead.
Absci claims to have a better formulation, I don’t know if that causes better results.
I hope they are successful but timewise they are so far behind that I actually wonder why they would pursue the same avenue as HopeMed, maybe even causing IP-issues?
If Absci really made their own research and found convincing results in their antibody then I am actually hopeful that Hopemed is good too.
Not sure if hopemed is still in the game, the p2 in china finished last year I think. And the p1 results weren’t that good.
https://www.hairlosscure2020.com/hope-medicine-raises-56-million/
Yeah, hope that’s still true. They are quite slow at least.
https://x.com/hairypapasmurf/status/1935389847234978122
Yeah that’s basically what I said.
They are not similar, it’s the same exact picture.
It’s about time. A startup revolution based on AI drugs is what’s needed, but the FDA will do everything in its power to keep things from moving forward.
On par with Minoxidil isn’t exactly getting me out of bed, but the potential to white hair is very intriguing.
Hello, kindly please do you mean it will help get rid of white hair?
Cautiously optimistic for this one. Total funding since their inception is 222 million USD.
The public comparison with HMI-115 is somewhat brutal and hints that it is a failure.
If they can start trials then we have another player. Fingers crossed.
To my eye the comparators are all about things other than effectiveness that matter to a drug manufacturer: stability, potential solubility in carrier solutions, potential for topical rather than intradermal injection application, etc.
Typo Admin: “Absci received a $20 investment” should be $20 million.
Thanks Yoda!
We know more than we ever knew about baldness, how long do we still have to wait for a cure? Is it always 5 years away? I’ve been waiting these 5 years for 30 years.
I honestly thought Follica – which developed the most efficacious protocol since finasteride (44% regrowth compared to baseline) – would have been on the market by last year. And instead, after decades of development, Phase I-II completed, retail-facing website and clinic recruitment, and it’s gone.