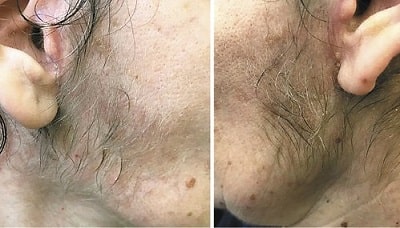
Update: July 2022 — Systemic review of hair loss after Covid-19.
The biggest news in the hair world for the past eight months has been Covid-19 causing rapid hair loss and shedding. This side effect is especially common in women. My Google Alerts are frequently filled with news on Covid-19 (aka Coronavirus) and hair loss. The latest came yesterday, courtesy of NPR.
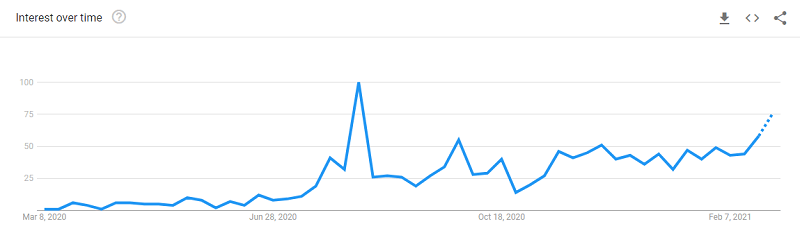
Does Covid-19 Cause Hair Loss?
According to the official statement from the American Academy of Dermatology:
“Fever is a common symptom of COVID-19. A few months after having a high fever or recovering from an illness, many people see noticeable hair loss.”
This type of rapid and sudden hair shedding is known as telogen effluvium. The unfortunate thing is that the shedding often happens two to three months after Covid-19 (or any fever or illness). And the shedding can last for six to nine months.
It is emotionally draining to see handfuls of hair on the brush and in the shower on a daily basis. Especially if you have never had significant hair loss in the past. Most people who get the Coronavirus do not get tested for it right away (if ever). So oftentimes, one can never make a conclusive diagnosis as to the reason behind one’s sudden and drastic hair loss.
Actress Alyssa Milano was probably the first celebrity to discuss this traumatic post-Covid hair loss issue. In August 2020, she posted a viral video on her hair loss, months after getting infected with Covid-19. Thousand of women (and some men) have since posted videos on social media showing fistfuls of hair coming out while brushing or combing.

Other Reasons: Stress and Vitamin D Deficiency
It should be noted that telogen effluvium can also be caused by excessive emotional stress. During this past year of the pandemic, global stress levels have skyrocketed. Women with children likely suffered even more. Domestic abuse cases and homicides also went up significantly in the US in 2020. Perhaps the only group that might have experienced reduced stress levels are the highly introverted who enjoy spending significant solo time indoors.
The good thing about stress induced hair loss is that the hair almost always comes back after some months. Unfortunately, it is almost impossible to reduce stress levels when one is shedding hair profusely on a daily basis. Doctors recommend meditation, nature walks and various other relaxation methods to try to stop the vicious cycle. Recovery can be slow, and hair loss treatments such as PRP can only help slightly.
Another lesser discussed issue is Vitamin D deficiency related hair loss. Most people in the developed world spent an unnatural amount of time indoors during this past year of lockdowns. This reduced daily sunlight exposure levels tremendously. Especially in the winter months, when one has a limited window of opportunity to get some direct sunlight.
Expose to the sun’s rays is the only natural way for our skin and body to produce Vitamin D. I take Vitamin D supplements in winter, but still try to make sure that I get direct sunlight exposure for at least a few hours each week. Scientists have even found that low Vitamin D levels increase a person’s chances of dying from the Coronavirus.
Excessively low vitamin D levels weaken our immune system and cause a host of other health issues in the long run. A 2020 study from India found that 86 percent of men with androgenetic alopecia had low serum vitamin D levels. For those with darker skin, it is even more difficult to get appropriate levels of vitamin D from sunlight.