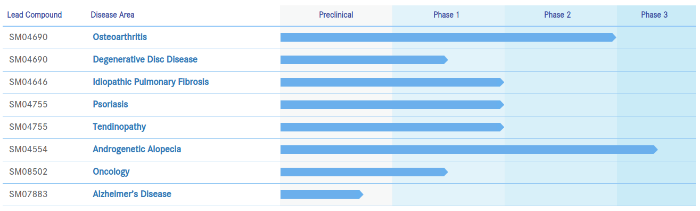
I have covered Samumed (US) close to 20 times on this blog. In 2018, the company started Phase 3 clinical trials for its androgenetic alopecia (SM04554) topical product.
Can Samumed Release Hair Loss Product in 2022?
Yesterday, Samumed’s CEO Dr. Osman Kibar gave an interesting interview to Fierce Biotech (h/t reader “John Doe”). The most relevant part of this interview for us is:
“Its alopecia treatment is close behind, with approval anticipated in late 2021 or early 2022.”
Not a surprise as far as time frame goes, but Mr. Kibar seems confident that they will get approval. I am assuming he agreed with the above sentence in the interview or was quoted as such.
Not sure if other CEOs “anticipate” approval chances before Phase 3 trials are complete. I am hoping that Samumed has seen good results during the first year of ongoing Phase 3 trials in order to warrant this confidence.
The interview also has some interesting information about the scientific reasoning behind why Samumed’s products are safe despite targeting the critical Wnt pathway.
Hopefully it won’t cost 200k.
More important than the announcement of the product in 2021 /
What is the success rate of treatment and how effective?
Many treatments are waiting for them after years without success .
still 2 years away…
I was wondering the same thing. What are anticipated results?
From the phase 2 trial, both doses (0.15% and 0.25%) had about a 10% increase after 3 months. I don’t know if they’ve changed their vehicle but the dose has remained the same. This looks like another minoxidil but different mechanism, so it can be stacked. And if Breezula gets released in 2022, looks like we’ll be on ‘The Big 5’ then.
Supergate, appreciate the explanation. So it’s one more mildly effective solution to add to the cocktail. Thanks!
Like other products it will Just be as efficient than monixidil.
So disappointed…
FDA Approval in 2021 or 2022, since they’re doing the clinical trials in Turkey, I wonder if SM04554 could possibly be available in other countries prior to the USA release?
Previous trial was applying for 90 days and then seeing results at 6 months. Current trial is applying for a year straight.
Can someone please remind me – is Samumed a lotion that you rub on your head?
I don’t care if it’s a needle up the ass. If it works I will take it :-)
You don’t count.
You’ve taken things up there.
But I bet if it worked I would have the rest of you doing it. Or just me saying it worked without producing any evidence at all.
Don’t get it…
Of finasteride does cause cancer than Merck should be facing more law suits.
It’s a lotion.. hopefully it doesn’t destroy texture and feel like minoxidil’s fingernails scratching a chalkboard? It should be fine since it is a small cell signaling Molecule.. Hopefully Turkey approves it? FDA gatekeepers are flat out anti American with their bs forever trials.. hopefully Trump jerks a knot in somebody’s ass Over at the FDA cabal or maybe just fire them all and get some honest people that can do a job post haste?
Good news I guess for 2021. The better news will be if it can grow some legit thick regrowth. I’m taking 20% + more density and halt of fuether loss long term 5 plus year like finasteride. If it works only for 6 months like CB then downward decline then f it….
@yoda: fantastic question.
Congrats to all the younger victims of this disease ( honestly im not being snarky), you have a plethora of upcoming treatments to look forward to. You still may have a chance to live the majority of your life not having to endure what earlier generations have. Kids that are 10 now may never have to worry about balding at all.
@Champpy they aren’t here yet and let’s not forget shiseido has disappeared at the last minute
Is there concrete news with shiseido or just delays as usual ?
Would be perfect to have the price of tsuji’s treatment decrease, or at least to have a less effective solution but at least we can wait.
Will the treatment arrive in both Turkey and US markets at same time?
So this is how Medipost NGF-574H looks like. … I wonder if this works and when we can buy it.
https://www.youtube.com/watch?v=SUbtQM71oCc
Yes! This is a big one, I have high hopes and expectations for this..But then sometimes I think I’m being naive
Should know by end of Oct, according to Medipost results in 3 months akin to Minox!
What is this medipost all about?
If there is no shed + an easier method of application, it might be a better alternative.
I am still sceptical concerning a possible cancer risk of the Samumed approach.
As we have known for a long time the WNT-pathway is a main driver of cancer (a recent study can e.g. be found at https://www.ncbi.nlm.nih.gov/pubmed/28647886 ).
According to the Fierce Biotech article “Samumed’s drugs target CLK and DYRK, kinase enzymes that are upstream of the Wnt pathway.”
But also CLK is involved in cancer development, so CLK-inhibitors are a promissing anti-cancer strategy (a recent study can be found at https://www.ncbi.nlm.nih.gov/pubmed/29769258 ). The same is true for DYRK: https://www.ncbi.nlm.nih.gov/pubmed/28554575
So in my opinion it is still unclear, if Samumed´s approach is safe enough to get an approval.
Best regards
Andreas
You’re perhaps right. Unfortunately the fda testing for this compound is only 3-4 years. We may not know of long term effects i.e. cancer. When altering a physiological function of pathway for stem cells, I’m quite concerned of a mutation that can occur.
However remember, most people take finasteride even with a correlated 20% occurrence of a rare type of prostate cancer. So, essentially people don’t care, until s**t hits the fan.
Hi, where do you get that 20% figure from? I thought the main “problem” with Finasteride is that it can mask the symptoms of prostate cancer; though if the fact that one is taking the drug is taken into account with the results then that should not prove such a problem (?) In my case I recently gave up on the drug. After 2.5 years it is clear it is not working for me. Bit of a relief in one way as I am not keen on taking any sort of drug on a long term basis. I have come to the view that my MPB is so aggressive (albeit slow progressing) that only some sort of hair multiplication or stem cell technology whereby new follicles are implanted will work for me. Some way off, but am counting the days (!)
I’d have to find the study again. However correlation does not mean causation. Also most, not all folks taking finasteride don’t realize this drug is altering DNA transcription/translation factors as it’s a steroidal hormone. Doctors whom prescribe this drug should properly educate potential users. It can be explained in layman terms in 5 mins. For those that don’t care, go ahead, if hairloss is detrimental to your life. Even though I’m very optimistic for Breezula, I have to keep in mind, that it has risks even though it targets only to applied areas. It has a different mechanism but it’s still interfering with the steroidal hormonal part of the endocrine system like finasteride.
No. Finasteride is associated with a major decrease in prostate cancer.
I don’t think most people in this forum would care about that risk. Most would happily take any drug, despite any risk, in the promise of results. If results are definite then that’s even more attractive.
Actually I think Propecia doesn’t cause cancer it can disguise a cancer of the prostate during diagnosis. I don’t think Propecia itself is the cause of cancer since the drug itself is designed to lower the chances of prostate cancer.
However, I can see samumed being a concern long term via wnt. It would be something to Def investigate further.
A high intake of meat and dairy leads to cancer. Smoking linked with lung cancer, tanning salons linked with skin cancer, alcoholic mouth wash linked with lip cancer, etc. So for most people (Westerners) their cancer risk card is marked anyway. This reason alone will not stop people if the treatment works, unless it is so high risk the law prevents its release.
Depends on the Quantity and Quality of hair growth.
I think Summer 2020 when Aclaris releases its results of a high dose of JAK will be very interesting. In the very least choices and competition will keep prices lower than they would be otherwise.
NASA jak. Off
It doesn’t work – drop it
Topicals all difficult to apply remember, that’s the toughest part in this.
Okay, no offense. Topicals are easy to apply, unless you are paralyzed, missing a limb, etc. it’s a regimen that requires discipline. Heck if I found a topical compound that caused 99.99% (*nothing is 100%) regrowth that must be used everyday. I’m certain a very high percentage of bald/thinning people (not disabled) will use this treatment religiously and not complain.
A daily topical with a 99% regrowth rate is a dream situation!
Totally agree with you nasa, Aclaris I believe is the most solid contender of them all. Looking forward to the 12 month results with just a .46% of a dose. Higher dose trial shall be fascinating to look forward to! Great times ahead for Jaks
Nasa, there is no trial for aga as of now with Aclaris. Didn’t they say they are looking for another company to run the trial? As of now nobody picked it up to run with it. Maybe I am mistaken and the phase 2b or whatever it is is currently happening. Can anyone confirm this ?
Trials have been successful. They just need partner for final expensive stage, which they will get soon.
https://www.hairlosscure2020.com/aclaris-announces-positive-6-month-results-for-androgenetic-alopecia/
Admin, All of our hopes and dreams have been answered by “kimchi drink”!
Or, it’s a practical joke designed to increase sales until we figure out it’s useless :-/
Where do I get “kimchi drink”? Lol
Can anyone give me a simple, easy-to-understand summary of potential, upcoming treatments / cures?
Tsuji — They clone your hair and implant the cloned hairs into your scalp. So it’s not a traditional transplant.
Samumed – A lotion for your scalp.
Replicel —Similar to PRP
Am I roughly on the right track? Any other big contenders?
Aclaris & other companies – JAK lotion. This is a big possibility and we will know next summer when their high dose JAK study is released with photos.
If you think the jak pics are impressive you have low low standards .
They picked their two best responders and you feel it’s a home run !
Watch out ladies – nasa and company are coming at you with 3 extra strands!
The first trial was at a LOW dose. They plan to up the dose for the second dose and no gurantees but I think it will replace HT’s and hopefully even better.
Ever run your car on very low Octane gas then up it to super high dose BIG Difference and we will find out in only another ~9 months.
Seriously??? Stop with the fanboy stuff. Comparing an running an engine with someone’s health. Utter insanity
I wish I had my hair back … the stress of having to fix my hair with hairspray and various rubbish is something I hate. the best solution would be a shampoo … no needles or stem cells …
@admin – perhaps start a poll to measure people’s importance of hair loss. The questions being your age and would you halve the rest of your natural lifespan for a permanent NW1.
I think it would be interesting to see how far people would go.
invitrohair guys?? Just filed a patent
Indeed, there is a ruckus on hair loss forums concerning invitrohair.
@admin can you check further into this?
huh
https://www.bbc.com/ideas/videos/why-baldness-is-so-hard-to-cure/p07ktlby
thanks bud. That guy sums it up perfectly!
In some respects probably a more realistic perspective than we would like to believe. From how desperate we are for a cure creating an environment ripe for fraud to how willing we are to believe that any new legitimate scientific discovery means a cure is just around the corner. However, I’m not sure how up to date his research is given that he states we were born with all of the follicles we would ever have. That thought was disproved as far back as 2006.
Thanks for sharing lorence
What do you think about regenera activa ?
Is that a type of yogurt?
Ha ha!
Hahahahaha
Admin did you see the news about follica? Phase three and fda filing in 2020?
I am interested in learning more of Regenera Activa as elk.
@ Admin..Admin can u plz do the necessary in regards of Invitrohair/Hairoid
Samumed, please please please apply for FDA clearance. By chance, if your SM04554 is weaker then minox, that’s fine, I’ll still be happy as it least it’s something myself and many others can utilize. Minox is a no no for many of us due to facial bloating, wrinkles, and lowering of blood pressure. Please samumed, dont get intimidated by comparing your product to fin or minox. Please!:)
Hello,
Does anyone have any news on the release date of SM04554, any forecasts?
Já estamos em 2022, cadê o produto? É só bla bla blá como sempre tem sido…