I have covered Samumed and its SM04554 compound to treat hair loss dozens of times in the past. The company’s Phase 3 Trials end in 2020.
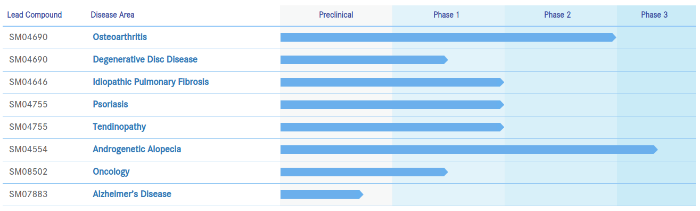
Samumed is aiming to treat numerous medical and cosmetic conditions via activating and/or modulating the Wnt signaling pathway. Of all those targeted conditions, androgenic alopecia is the furthest ahead and the only one that is currently in Phase 3 Trials:
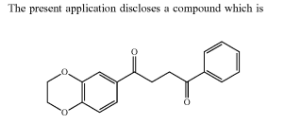
Samumed Compound Structure
A few days ago, a reader named “Thomas” emailed me about a new Samumed patent (Publication date = November 22, 2018; Filing date = December 27, 2017).
Thomas pointed out something very interesting. For the first time ever, Samumed has published the chemical structure of its main Wnt/ß-catenin signaling compound. Right under the section titled “Abstract” in the above linked new patent.
Samumed has a decade-long online trail of numerous filed patents, with the vast majority of them including the names of their key scientists John Hood and Sunil Kumar. I have not tried to go through all of these online patent documents, especially those not focused on androgenetic alopecia (male pattern hair loss).
So I am not certain if the above chemical compound structure image is the first time that it has ever been released. It is also not clear if all of Samumed’s products will be very close in structure to the above compound image. The company has published compound images in the past that look very different from the above (e.g., the one in here for cancer treatments; another in here for Indazole-3-carboxamides and their use as Wnt/β-catenin inhibitors and more).
It would be useful if readers (especially the Chemistry experts on here) provide relevant feedback to this post. For example:
- What does this compound’s molecular structure mean and how is it unique from other compounds (Samumed ones and non-Samumed ones) that modulate Wnt/ß -catenin signaling?
- How does this latest compound image differ from other related compound images that Samumed has published in the past?
- If this really is the first time that this particular image has been released by Samumed, why so late in the process?
