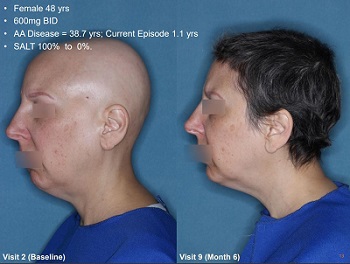
Update: July 2020 — Unfortunately, Aclaris ceased work on JAK inhibitors and androgenetic alopecia in 2020. Moreover, new findings from July 2020 suggests that JAK inhibitors do not help AGA sufferers.
Update: Dr. Neal Walker interview.
Note: Some great photos are in their investor presentation from today. Pasting a couple below after getting permission.
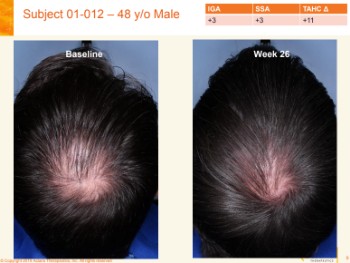
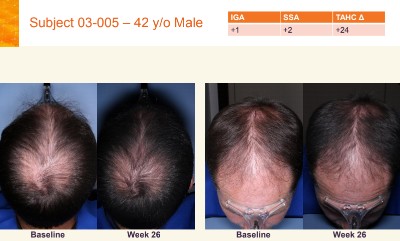
Aclaris Topical JAK Inhibitor Works for AGA
Huge news from Aclaris Therapeutics. They have just announced that their much anticipated topical Janus Kinase (JAK) inhibitor product (ATI-502) gave positive hair growth results in patients with androgenetic alopecia (AGA).
The results are for their interim 6-month Phase 2 open-label clinical trials of ATI-502 (also called AGA-201) in humans. 22 males and females participated in the trials till completion, including hair growth measurements. They applied ATI-502 to their scalp twice daily for 26 weeks.
Investigators rated 73% of subjects (16/22) as experiencing increased hair growth, and a higher 82% of subjects thought they saw increased hair growth through self-assessment.
For a long time, many people did not believe that JAK inhibitors could result in hair growth in patients with androgenetic alopecia (i.e., male pattern hair loss). All the results in humans to date had only shown efficacy in patients with alopecia areata (AA), an inflammatory rather than hormonal condition.
Aclaris JAK Inhibitor Trials to Continue
I may add more to this post later, but for now the most significant quotes:
“The overall change was an increase of 8.6 hairs/cm2. Target area hair count increase was 15.3 hairs/cm2 in female subjects and 5.6 hairs/cm2 in male subjects.”
Per Aclaris CEO Dr. Neal Walker:
“This finding demonstrates that inhibiting a non-hormonal and inflammatory-mediated pathway may be an option for the treatment of AGA.”
“Through recent formulation work, Aclaris can achieve significantly higher topical concentrations of ATI-502.”
There was one “unrelated” case of breast cancer among the trial patients, but no-one else had any adverse side effects. I am sure everyone will look in detail at the side effect rates when 12-month trials are completed.
In the first half of 2020, Aclaris plans to initiate a double-blind, randomized, controlled Phase 2 dose-ranging clinical trial with higher concentrations of ATI-502. “With potentially, a female focus.” I hope that just means more females than males, and not just “only” females.
I do not think that this will be a cure for male and female pattern hair loss by any means. Perhaps a highly effective treatment in some patients. Moreover, for those of us who have major itching associated with our hair loss, perhaps we also have an inflammatory component to our hair loss as I have postulated in the past. Maybe the itchiest patients will see the best results?
Worth checking out ACRS stock price movement today.
Congratulations to the biggest JAK inhibitors for AGA fan of them all: blog reader “nasa_rs”.