Perhaps the best hair loss cure related news of 2023 just came out last week (h/t “Theo”). South Korea based Kangstem Biotech plans to release a hair cloning type of hair loss treatment in 2024.
They will commercialize a cloned hair follicle-based drug screening and efficacy evaluation method; and begin nonclinical efficacy evaluation of hair transplants based on the cloned hairs.
Kangstem Biotech to Commercialize Hair Cloning Treatment in 2024
Kangstem Biotech (South Korea) was founded in 2010 by Kyung-Sun Kang and is publicly traded. Its shareholders include a range of major Korean and Western companies. Without checking this company’s reputation, I might have delayed this post to next year.
The company specializes in cord-blood derived stem cell and other anti-aging related treatments. They are also a contract development and manufacturing organization (CDMO). I never heard about them till this week.
During the past two weeks, Kangstem Biotech had two press releases that are quite remarkable:
- On December 6th, the company announced plans to speed up the commercialization and launch of skin organoids to 2024. Interestingly, they had an update about their artificial skin technology and Seoul National University partnership in 2021.
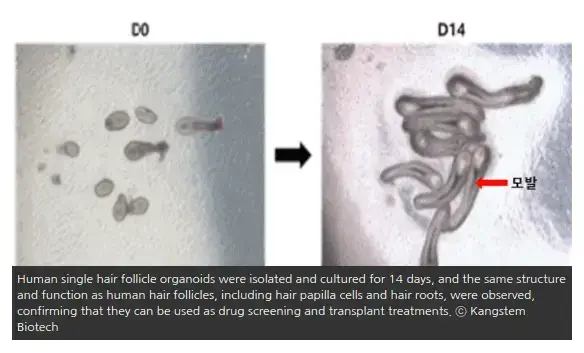
- On December 22nd, the company announced plans to begin commercialization of the world’s first human hair follicle organoid-based hair loss treatment in 2024.
Per the second press release, Kangstem Biotech signed a contract with the Seoul National University Industrial Cooperation Foundation for:
“Human hair drug screening and human hair follicle production and culture technology for hair transplant materials to develop and commercialize hair loss treatment based on hair follicle organoids.”
Also check out the summary in Newsprime. And on Linkedin.
The company also states the following per the Korean to English translation:
“This technology is the world’s first artificial production of human hair follicle organoids in a test tube, and is a technology that reproduces human hair follicles.”
Kangstem has a two pronged approach when it comes to usage of its technology:
- Provide a drug screening platform for the development of hair loss or hair growth pharmaceutical treatments. They plan to launch their business for hair follicle-based efficacy evaluation methods in 2024.
- Begin non-clinical efficacy evaluation of using the hair follicles they culture for use in actual hair transplants. Also in 2024.
The government regulations for regenerative medicine in rapidly aging developed Asian countries have become very flexible. South Korea cannot afford to wait too long, considering that average birth rates in the country hit just 0.72 children per woman in 2023.
Also of significance, South Korea and Japan are both trying to become world leaders in cosmetic procedure related tourism.
South Korea’s Leadership in New Hair Loss Treatments
This adds yet another new South Korean entrant in the hair cloning or hair multiplication sector. Others that I have covered recently include Epibiotech and Han Bio. For a list of all major South Korean entities that are working on any kind of important hair loss treatment, check out my page on hair loss cure research around the world.
Update: Below is an e-mail update to me on 12/29/23 from “Theo” —
“From the press release I understand that they will commercialize hair follicle/skin organoids for drug testing by the first half of 2024. In parallel they will test the hair cloning technology in non-clinical trials to confirm efficiency (if I understand correctly). This technology is similar to Stemson, because both companies works with iPSCs and in vitro, and Stemson is at least 10 years away from commercialization.
Non-clinical testing in South Korea is the first step required by the state, and then comes clinical trials.
Guidelines for clinical trial approval (CTA) for drugs:
https://credevo.com/articles/2017/09/25/south-korea-clinical-trials-regulatory-process/
This is a very well established company, very well funded, with high quality infrastructure and connections. So it should go very fast.
The main CEOs and scientists in KangStem come from Seoul National University, which is in the top 25 universities worldwide.”