We regularly hear about research involving hair growth in rats, mice, rabbits, hamsters and macaques. However, I have never read about scientists managing to successfully grow human hair in pigs. Until now.
Note that pig (porcine) skin has striking similarities to human skin. Thanks to reader “Bryan” for first posting about the below new study.
Hair Growth in Pigs from Human Dermal Papilla Cells
In April 2023, Epibiotech CEO Jong-Hyuk Sung co-wrote an interesting letter in Experimental Dermatology. It entails his team’s success in growing hair in pigs via the injection of human dermal papilla cells (hDPCs). The initial phase of this work on pigs was also discussed in Epibiotech’s past updates.
Note that Mr. Sung has published a number of important hair related studies for more than a decade. In November 2022, I covered his highly interesting new summary paper on effective cell therapy for hair regeneration. He even responded to some reader comments in that post.

Coming back to this latest paper, the conclusion was that intradermal hDPC injections in pigs increased the hair follicle number, thickness and angiogenesis. Moreover, a single injection of hDPCs seems to be effective and survives for more than 3 months in clinical study.
Key quote (slightly reworded):
“Though numerous past research findings indicate the hair growth promoting effects of human DPCs in rodents, preclinical or clinical application alone has not been reported in pigs until now.”
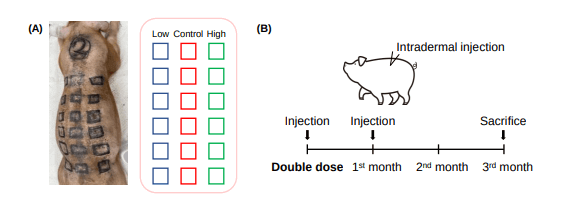
The research team injected hDPCs into the backs of three pigs across six sites. After just one injection of hDPCs, hair follicle numbers increased for three months. And hair follicle thickness was increased for one month.
Interestingly, there was no difference in hair follicle quantity and thickness between low- and high-dose delivery of human dermal papilla cells. Also of note, the number of microvessels around the hair follicles was increased in the hDPC-treated group.
Normally (after 10 years at this), I would not write an entire post for such a one-off animal model study. However, two factors made me reconsider:
- The involvement of Epibiotech’s CEO in the research.
- Considering the fact that this is the first ever report on human dermal papilla injection spurred hair growth in pigs.
Also of note, this research was funded by the South Korean government via the Korea Drug Development Fund and the Korean Fund for Regenerative Medicine. Yet more reason to trust the legitimacy and reputability.