I have covered PolarityTE (original ticker symbol “COOL”) and its SkinTE product a number of times on this blog in the recent past. Make sure to read my original post about the company from last year.
The SkinTE product is meant to regenerate skin and help burn victims and others with damaged skin. However, there is also a potential application for a hair growth product down the road. For more on the latter, read the second half of my post from earlier this year.
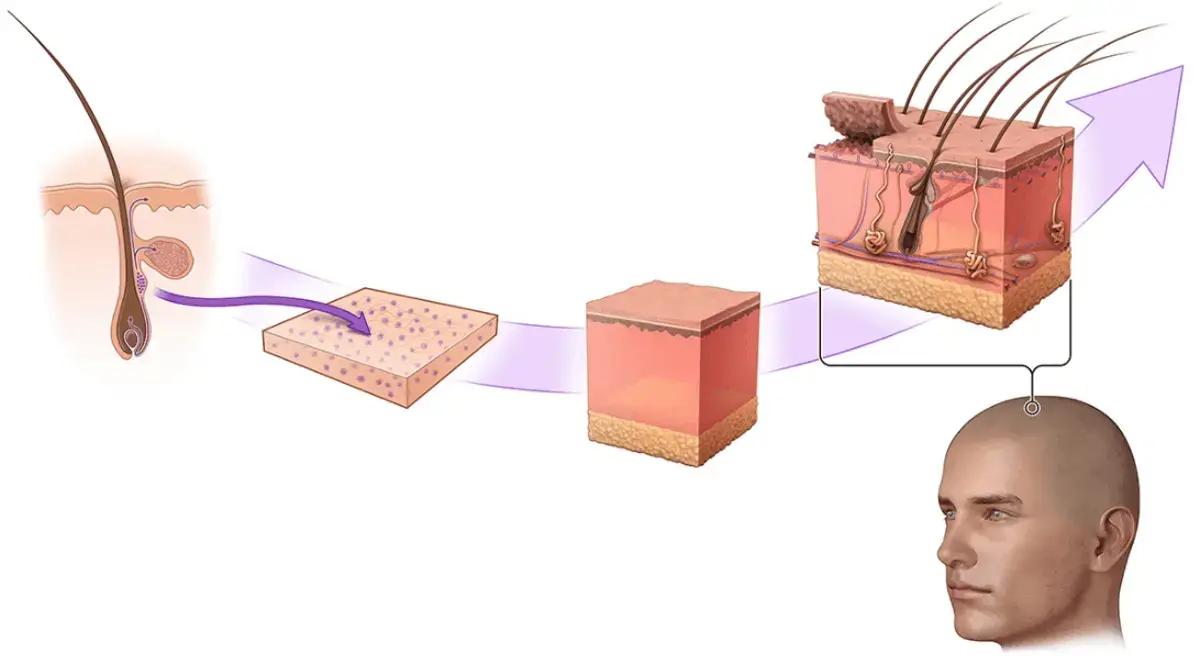
PolarityTE has always maintained that its SkinTE product can regenerate damaged skin that also comes with intact new hair follicles. They even updated their site with the below image of a balding head a few months ago. In addition to mentioning the potential future application of SkinTE to treat hair loss.
PolarityTE Gets Positive Feedback from Test Patient
Earlier today, I got an e-mail from PolarityTE with a link (now dead) to an update. Apparently, they recently tested the SkinTE product on a patient with major leg wounds from a motorcycle accident. The patient, named “Devon”, is extremely happy with the results. The money quote is below, with the key part in bold:
“My leg feels as close to normal skin as I can imagine, and there is even some hair growth. If I had to choose between SkinTE and another skin graft, I would choose SkinTE every time because it is actually working.”
If “Devon” is definitely not imagining things, this is extremely encouraging. Only one data point of course, but well worth a new post on my part.
As I have mentioned in the past, the PolarityTE team is composed of high quality experienced scientists who have decades of experience working in renowned plastic surgery centers. The company has few problems in getting new funding; has been expanding its facilities in Utah; and CEO Denver Lough keeps getting great media coverage.
As if all the above is not enough, the SkinTE product represents an autologous therapy, which means that it will not require extensive clinical trials. In fact, it is already being used in various clinical settings from early adopters as this latest update mentions.