I cover Stemson Therapeutics (or its founder Dr. Alexey Terskikh, or its CEO Geoff Hamilton) at least several times a year. This company represents the leading hope for a hair multiplication related hair loss cure. Among the highlights:
- In 2015, I covered Dr. Terskikh and his team’s success in growing human hair in mice.
- In 2017, I interviewed Dr. Terskikh.
- In 2018, I discussed the at-the-time newly registered company Stemson Therapeutics.
- In 2021, I covered an interview of Geoff Hamilton.
I now just update this comprehensive Stemson post twice a year rather than write a separate one each time. This is because Google Indexing tends to ignore short update posts.
Update: March 13, 2024
Great new interview with Geoff Hamilton. The Aderans injection is most likely a one and done procedure! Or at least very long-lasting. Moreover, Stemson might go straight to Phase 3 trials if they deem the old Japanese findings sufficient enough.
As expected, the Aderans method will not bring back hair in totally bald scalps. That is only going to happen when Stemson’s own iPSC based approach comes to market. And they will need to raise 10s of millions of dollars in funds to get those trials going till the end. They are also open to working in other countries with more favorable regulations.
The guy walking in at 1:50 looks like Kevin D’Amour. Who I covered in my February 10 update further below!
Update: March 9, 2024
Stemson Therapeutics and Aderans Announce Licensing Deal
Earlier today, Stemson Therapeutics (US) and Aderans (Japan), the parent company of Bosley® and HAIRCLUB®, announced a partnership agreement (licensing deal).
This arrangement secures Stemson the exclusive global rights to research, develop and commercialize hair regeneration therapeutic products based on Aderans’ proprietary hair regeneration cell therapy technology. I cover all the details in my decade old disappointing post about Aderans’ hair multiplication failure. Now reinvigorated.
Update: February 10, 2024
Excellent detailed and very scientific interview with Stemson chief scientific officer Kevin D’Amour. The focus is on iPSC-derived cell therapy in the hair loss space.
Update: February 6, 2024
Stemson Therapeutics Announces Technological Breakthrough in New Hair Growth
Good news. Stemson Therapeutics just announced a major technological advance that will enable its proprietary iPSC-derived hair rejuvenation solution to advance towards human clinical trials. Full press release is here.
Key quote from CEO Geoff Hamilton:
“Stemson’s engineered follicular units will be the first therapeutic solution capable of replacing lost hair follicles. The recent demonstration of robust and reproducible all-human hair growth in humanized mice is a strong indicator of potential success in human trials.”
Outside of Japan’s OrganTech (Dr. Tsuji), Stemson is the most important and promising company when it comes to the realization of a hair loss cure. Perhaps Shiseido (Japan) and a host of newer South Korean contenders could yet surprise us. The main advantage that Asian companies have is the potential for much faster human clinical trials due to more favorable regulations than in the US.
Update: December 1, 2023
There is a brand new informative video presentation from Stemson CEO Geoff Hamilton (h/t “Ben”). The clinical time frame is still slow, with human trials only starting around the end of 2025. I wish they had done these in Asia or even the UK, where reguations are more favorable. The video elaborates on both their autologous and allogeneic cell therapy products.
Stemson Therapeutics: 2022 Updates
Update: December 30, 2022 — Stemson’s Director of Histology and Pathology Dr. Allan Dovigi wrote an interesting post on Linkedin that has so far received only one comment. I like his enthusiasm on the speed of progress in organ regeneration and medicine in general. Key quote with slight changes by me:
“At Stemson Therapeutics we are growing hair follicles for transplantation in our patients with alopecia. We are removing the barriers to the cell’s genetic programs and switching them back on to allow regeneration of the original tissues and anatomy. I can observe all the histology of the regenerated hair follicles, epithelial root sheath around hair shafts, dermal papilla, sebaceous glands and pilar erector muscle. I find it simply amazing. Peering through my microscope has never been more exciting.”
It is very interesting that he mentions the crucial to hair (but usually ignored) arrector pilli muscle. He predicts that humans will be able to regenerate entire organs going forward, so organ transplants will become obsolete. Note that Stemson currently has 25 employees with Linkedin accounts.
Update: November 20, 2022 — VP Meghan Samberg presented on Stemson’s iPSC-derived cell therapy approach for hair follicle regeneration at the World Congress for Hair Research in Australia.
Update: October 25, 2022 — Interesting new interview with CEO Geoff Hamilton. Key quote:
“We’re trying to get our first product into clinic, which is an autologous cell therapy-based approach to bioengineering hair follicles. It is probably going to be at least a couple of more years before we feel we’re really ready.”
Update: February 12, 2022 — Joe Tillman and Spencer Kobren have released three mini-interview videos of Stemson CEO Geoff Hamilton. I am embedding Part 3 below:
Update: February 1, 2022 — Stemson just hired Dr. Kapil Bharti and Dr. George Murphy to its scientific advisory board. Both seem to be renowned scientists in the world of regenerative medicine and stem cell research.
Update: January 18, 2022 — Mr. Hamilton is covered in a new MIT Technology article titled: “Going bald? Lab-grown hair cells could be on the way.”
Alexey Terskikh and Geoff Hamilton
Update: December 2021 — Stemson founder Alexey Terskikh just co-authored an interesting new paper titled “The rise of induced pluripotent stem cell approach to Hair Restoration.”
“Combined with robotics and automation of the transplantation process, this novel regenerative medicine approach is well poised to make hair restoration a routine procedure affordable for everybody who can benefit from it. At present, it seems that the generation of DP cells through the neural crest intermediate has the lowest barrier to enter the clinical trials in the next few years.”
Note that Dr. Hamilton also gave the keynote address at the recent Global Hair Loss Summit.
Update: October 12, 2021 — Presentation at the Alliance for Regenerative Medicine’s “Meeting on the Mesa”:
Update: September 1, 2021 — Market Knowledge podcast interview with Geoff Hamilton. Make sure to watch the video at the bottom of that post.
Update: July 15, 2021 — Stemson covered by their local San Diego ABC News affiliate:
Update: October 2020 — The ISHRS interviewed Dr. Alexey Terskikh on video.
Update: September 2020 — Stemson just got another $7.5 million in funding from Allergan and Fortunis Capital. h/t reader “James”. Quote from the CEO of Fortunis:
“Stemson’s novel cell therapy approach to treat hair loss has game-changing potential.”
CEO Geoff Hamilton said the following:
“Stemson has established the biological and technical building blocks which are needed to solve the problem of hair loss. A truly curative solution is now feasible.”
We have heard similar praise from a number of other company CEOs during the past decade. So no use in getting hopes raised this early in the process.
Note that Allergan is involved in the hair loss world via investments in a significant number of upcoming companies and technologies.
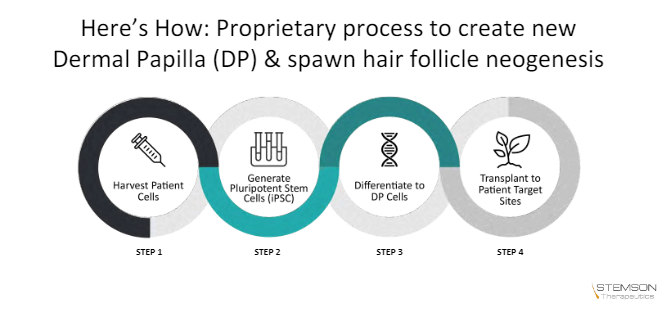
September 14, 2020
Stemson Therapeutics Patent Approved
Earlier today, Stemson Therapeutics announced that its cornerstone patent had received approval in the US.
This patent (No. 10716808) is titled “Methods and compositions to modulate hair growth”. The technology is licensed exclusively from the Sanford Burnham Prebys Medical Discovery Institute.
The process outlined in this patent entails a novel process to differentiate Induced Pluripotent Stem Cells (iPSC) into dermal papilla cells (DPCs). The latter cells control hair follicle generation and hair cycling.
Considering that Stemson is yet to even start human clinical trials, I do not expect this product to come to market any time soon. However, the scientific research behind this company’s technology seems to be solid, and the company itself is well funded (see further below).
On the company’s news page, last week they announced the hiring of Dr. Meghan Samberg as Vice President of R&D and Preclinical Development.
June 27, 2019
 During the last 30 days, we have received positive updates from: Aclaris Therapeutics; Dr. Lowry (new company Pelage Pharmaceuticals); Follica; Polichem; and Shiseido.
During the last 30 days, we have received positive updates from: Aclaris Therapeutics; Dr. Lowry (new company Pelage Pharmaceuticals); Follica; Polichem; and Shiseido.
We also had a positive story from Columbia University (Dr. Angela Christiano) this week that I did not cover. It pertain to past research on JAK-STAT signaling and hair regrowth. I did not think that we could have a more inspiring month than we have just had in the world of hair loss cure research.
Stemson Therapeutics has Arrived
Perhaps the biggest story of the year is the fact that Stemson Therapeutics is now a reality, and they have officially launched their website. I originally covered this company in 2018, and discussed its research all the way back in 2015.
Moreover, Stemson has secured a multi-million dollar investment from pharmaceutical giant Allergan. In Dr. Alexey Terskikh’s interview with me in 2017, he mentioned that the biggest thing holding them back was lack of sufficient funding.
I have asked Dr. Terskikh to give us another interview soon and hopefully he will accept. Earlier this month, he told me that his lab was given a podium presentation at the ISSCR conference on June 27th. However, he did not mention the below surprising development.
Functional Hair Follicles Grown from Stem Cells
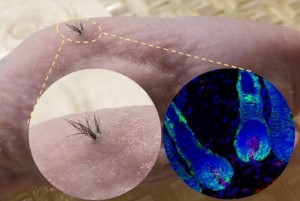
Earlier today, San Diego based Sanford Burnham Prebys published a what seems to be groundbreaking new article: Functional Hair Follicle Grown from Stem Cells. Note that this research institute is affiliated with Dr. Terskikh.
The breakthrough is because scientists from Sanford Burnham have created natural-looking hair that grows through the skin using human induced pluripotent stem cells (iPSCs). According to the article, this “could revolutionize the hair growth industry”.
Unlike in past experiments where new hair growth was underneath the skin, haphazard, and disorderly, the current results produced hair growing above the skin in a uniform pattern. The breakthrough was achieved by using a biodegradable 3D scaffold that guided hair growth through the skin in its preferred direction.
Actual paper is here. Lead researcher is a Dr. Antonella Pinto.
The current protocol is based on mouse epithelial cells combined with human dermal papilla cells. However, the derivation of the epithelial part of a hair follicle from human iPSCs is currently underway in the Terskikh lab. Key quote from the article from today:
“Combined human iPSC-derived epithelial and dermal papilla cells will enable the generation of entirely human hair follicles, ready for allogenic transplantation in humans.”
Make sure to read about the difference between autologous and allogenic. Stemson Therapeutics has licensed the above technology from Sanford Burnham.