Follicum
I am on Follicum’s mailing list and the company had four significant developments during the past month that were deemed worthy of e-mailing to subscribers:
- On January 18th, Follicum announced the identification of key receptors in human hair follicle cells to which the company’s lead hair loss candidate (FOL-005) binds. FOL-005 is a modified version of the endogenous protein, osteopontin.
- On February 2nd, Follicum announced the launch of its English language website. It is worth going through the menu titled “Hair Growth”. The Swedish version of the site remains online.
- On February 6th, Follicum CEO Jan Alenfall gave an interesting interview to Aktiespararna. He discusses both the hair loss product as well as the new diabetes product FOL-014.
- Of most important to us, on February 7th, Follicum announced that it had received go-ahead approval from the German Medicines Agency (BfArM) and German Ethics Committee. This is to commence a Phase IIa clinical trial in Germany in relation to FOL-005 on human patients. The company will partner with Clinical Research Center for Hair and Skin Science (CRC) in Berlin and bioskin in Hamburg.
Follica
In 2016, Follica announced that it was going to address its hair loss treatment via the acronym “RAIN”. At the time, I guessed the “R” to mean Regeneration, and the “N” to mean Neogenesis. Earlier this month, blog reader “PinotQ” notified us that Follica owner Puretech most likely recently updated its website and now spells out that the “AIN” stands for Abrasion Induced Neogenesis. Perhaps we just missed this development last year, but it is important enough to spell out here.
French Fries
A few days after I covered the groundbreaking work of Dr. Junji Fukuda, major newspapers around the world figured out a different (i.e., clickbait) take on the story. One that clearly got far more publicity and Facebook likes. They labeled Dr. Fukuda’s discovery as “Chemical in McDonald’s French Fries Could Cure Hair Loss” plus other minor variations of that title.
All because the chemical (dimethylpolysiloxane) used in the McDonald’s french fries via the oil fryers was involved in part of Dr. Fukuda’s research, even though it had no direct influence on hair regrowth. This french fries fable has since became the biggest hair loss related story of the year, and is unlikely to be surpassed in superficial importance for the rest of this year.
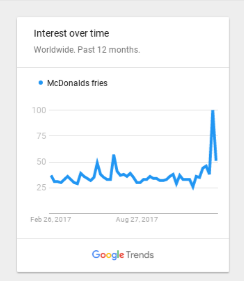
Unbelievably, numerous blog readers who already read my original post on this research still thought that the McDonald’s fries story was something totally different and perhaps worth looking into.
I had to delete the repetitive reader comments about this subject in recent posts and did not bother to respond to any e-mails abut this story.
The best advice comes from Dr. Fukuda himself:
“I have seen online comments asking, ‘how many fries would I have to eat to grow my hair?’” he said. “I’d feel bad if people think eating something would do that!”
Other Items of Interest
— Some new companies and increasing competition in the South Korean over-the-counter hair loss treatment market.
— Replicel gets new investment from Chinese company YOFOTO. Seems like the hair loss product related treatment rights were not granted, probably due to Replicel’s binding agreements with Shiseido regarding the Asia region?
— Since JAK inhibitors have been working on many alopecia areata and vitiligo patients, I have followed both conditions more closely in recent years. Here is an interesting alternative story about a vitiligo patient.