I covered Follicum (Sweden) and its FOL-005 (now FOL005) hair growth peptide in eight posts between 2015 and 2021. Prior to the company’s cessation of work on FOL005 and a 2022 acquisition by Coegin Pharma (Sweden).
Follicum’s unique osteopontin based hair loss treatment is very interesting. Their product can both stimulate scalp hair growth and reduce excessive body hair growth (hirsutism). And the same technology could potentially treat diabetes and inflammation related problems.
Follicum’s frequent press releases, CEO Jan Alenfall’s presentations, and the company’s rapid clinical trial progression impressed me. Their chief business officer Gunnar Gårdemyr was also in regular communication with me.
October 25, 2023
Coegin to Release FOL005 Hair Growth Peptide Gel in 2025
Some major new updates since I wrote the summary earlier this year (see second half of this post).
- October 17, 2023: Jens Eriksson becomes acting CEO of Coegin Pharma.
- August 31, 2023: Coegin shares a video update on its FOL005 development strategy.
- August 24, 2023: In its interim report, Coegin states the following (also see another summary here):
“After carefully analyzing the possibility of launching FOL005 as a cosmetic product line, we have come to the conclusion that it is an opportunity with great potential. This will minimize development risk, significantly reduce capital requirements and strengthen commercial opportunities in the short and long term. We have therefore revised our strategy and now aim to launch FOL005 as a cosmetic product series as early as 2025 with the USA as the first market.”
It seems like CosmeRNA started a great new trend. No more ten year clinical trials and failure to come to market!
- Follicum’s website seems to have been restored and updated very recently. The milestone plan page has a detailed chart for FOL005:
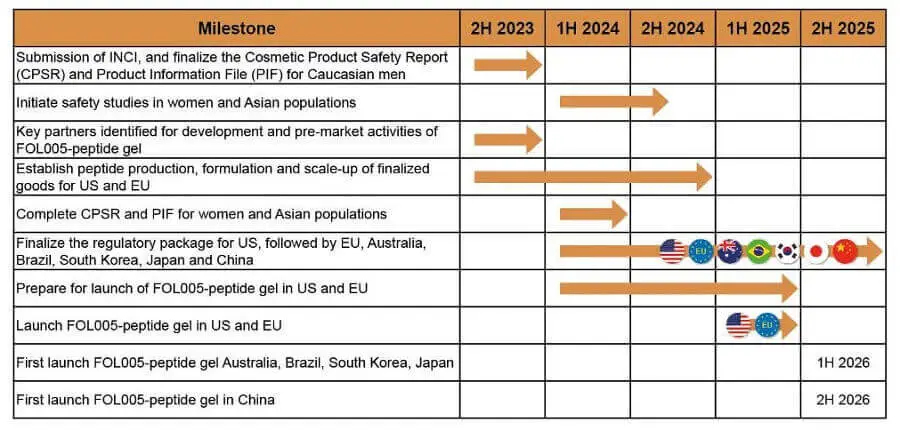
April 8, 2023
Follicum Past Clinical Trials
I covered both of Follicum’s past clinical trial results in 2017 and 2021, respectively.
- In 2017, Follicum’s Phase 1 clinical trial results were released. They were deemed as positive (8 percent increase in hair growth), with an effect comparable to that of Minoxidil. Moreover, safety was not an issue, with no major side effects in trial participants.
- In 2021, Follicum’s Phase 2 clinical trials of FOL-005 came out. The topical compound resulted in a 6.6 hair/cm2 increase in hair growth, but this was deemed to be insignificant compared to placebo.
Thereafter, the company ceased further development of FOL-005, and it all seemed over for yet another false flag operation. Or perhaps not?
Merger and Acqusition by Coegin Pharma
In September 2021, Coegin Pharma merged with Follicum. This merger was confirmed as an acquisition in 2022.
In the first of the two links above, Coegin’s CEO Tore Duvold said something of interest in the interview:
Question: “With regard to the hair loss project, which has recently been put on hold, how will it be handled once the companies merge”?
Answer: “Follicum announced that they have paused the hair loss project. In Coegin, we have strong capabilities in dermatology, and we will review and analyze the results before we make any conclusions”.
Follicum Back in the News
- In March, a Tweet from Coegin indicated that they were meeting with potential partners for Follicum’s FOL-005.
- Follicum was mentioned in February in an updated article titled: “Could We See a New Dawn for Hair Loss Treatments?”. It summarizes the problems that the company faced in trying to bring FOL-005 to market after the unimpressive Phase 2 trial results.
- When going through Coegin Pharma’s website, I found a highly detailed comprehensive corporate presentation on Follicum and FOL-005. It was presented at the end of 2022 by Coegin’s COO Kristian Lykke Fick.
Their take on the past trials is more positive. Key quote:
“FOL005 1.5% dose was on par with treatment effect reported for minoxidil and finasteride with a growth of 12 hairs/cm2 after 4 months of treatment. However, with more than 60% of subjects responding to treatment compared to competitors where 40% responders effect is previously documented after 4 months.”
Note that Follicum’s old Swedish website is being routed to the English site that has an expanded research section on FOL-005. In 2022, they also filed for a patent related to wound healing. I am surprised they do not mention anything in relation to their osteopontin-derived peptide’s body hair growth inhibition.
Follicum plans to conduct various new scalp hair growth related clinical trials from 2023-2027. On their website’s plans section, they state the following:
“We believe there is a high likelihood of success based on the previous clinical data.”
Too bad about the new time frame and the wait for venture partners for funding. Otherwise, I am not entirely pessimistic about this product. I do feel that it can compete with Finasteride and Minoxidil in terms of effectiveness, but via a totally different mechanism of action. And with no serious side effects.
